Page 605 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 605
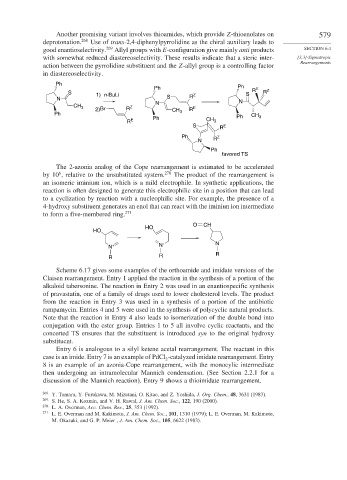
Another promising variant involves thioamides, which provide Z-thioenolates on 579
deprotonation. 268 Use of trans-2,4-diphenylpyrrolidine as the chiral auxiliary leads to
good enantioselectivity. 269 Allyl groups with E-configuration give mainly anti products SECTION 6.4
with somewhat reduced diastereoselectivity. These results indicate that a steric inter- [3,3]-Sigmatropic
Rearrangements
action between the pyrrolidine substituent and the Z-allyl group is a controlling factor
in diastereoselectivity.
Ph
Ph Ph R E Z
S 1) n-BuLi Z S R
N S R
N N
CH 3 Z E
2) Br R CH R
Ph 3
Ph CH Ph CH 3
R E 3
S R E
Ph Z
N R
Ph
favored TS
The 2-azonia analog of the Cope rearrangement is estimated to be accelerated
6
by 10 , relative to the unsubstituted system. 270 The product of the rearrangement is
an isomeric iminium ion, which is a mild electrophile. In synthetic applications, the
reaction is often designed to generate this electrophilic site in a position that can lead
to a cyclization by reaction with a nucleophilic site. For example, the presence of a
4-hydroxy substituent generates an enol that can react with the iminiun ion intermediate
to form a five-membered ring. 271
O CH
HO
HO
N + N + N
R R R
Scheme 6.17 gives some examples of the orthoamide and imidate versions of the
Claisen rearrangement. Entry 1 applied the reaction in the synthesis of a portion of the
alkaloid tabersonine. The reaction in Entry 2 was used in an enantiospecific synthesis
of pravastatin, one of a family of drugs used to lower cholesterol levels. The product
from the reaction in Entry 3 was used in a synthesis of a portion of the antibiotic
rampamycin. Entries 4 and 5 were used in the synthesis of polycyclic natural products.
Note that the reaction in Entry 4 also leads to isomerization of the double bond into
conjugation with the ester group. Entries 1 to 5 all involve cyclic reactants, and the
concerted TS ensures that the substituent is introduced syn to the original hydroxy
substituent.
Entry 6 is analogous to a silyl ketene acetal rearrangement. The reactant in this
case is an imide. Entry 7 is an example of PdCl -catalyzed imidate rearrangement. Entry
2
8 is an example of an azonia-Cope rearrangement, with the monocylic intermediate
then undergoing an intramolecular Mannich condensation. (See Section 2.2.1 for a
discussion of the Mannich reaction). Entry 9 shows a thioimidate rearrangement.
268
Y. Tamaru, Y. Furukawa, M. Mizutani, O. Kitao, and Z. Yoshida, J. Org. Chem., 48, 3631 (1983).
269
S. He, S. A. Kozmin, and V. H. Rawal, J. Am. Chem. Soc., 122, 190 (2000).
270 L. A. Overman, Acc. Chem. Res., 25, 353 (1992).
271
L. E. Overman and M. Kakimoto, J. Am. Chem. Soc., 101, 1310 (1979); L. E. Overman, M. Kakimoto,
M. Okazaki, and G. P. Meier , J. Am. Chem. Soc., 105, 6622 (1983).