Page 610 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 610
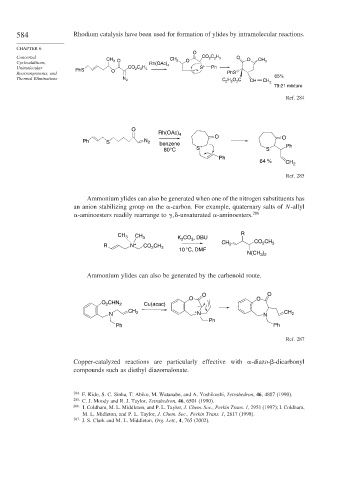
584 Rhodium catalysis have been used for formation of ylides by intramolecular reactions.
CHAPTER 6
O
Concerted CH CO C H 5 O O
2
2
Cycloadditions, 3 O Rh(OAc) CH 3 O – CH 3
Unimolecular PhS CO C H 5 4 S + Ph
2
2
Rearrangements, and O PhS
Thermal Eliminations N 2 H O C 65%
C 2 5 2 CH CH 2
79:21 mixture
Ref. 284
O
Rh(OAc) 4
Ph S N 2 benzene – O O
80°C S + S Ph
Ph
64 %
CH 2
Ref. 285
Ammonium ylides can also be generated when one of the nitrogen substituents has
an anion stabilizing group on the -carbon. For example, quaternary salts of N-allyl
-aminoesters readily rearrange to
-unsaturated -aminoesters. 286
CH 3 CH 3 K CO , DBU R
3
2
2
R N + CO CH 3 CH 2 CO CH 3
2
10 °C, DMF
N(CH )
3 2
Ammonium ylides can also be generated by the carbenoid route.
O O
O O
O CHN 2 Cu(acac) –
2
CH + CH
N 2 N N 2
Ph
Ph Ph
Ref. 287
Copper-catalyzed reactions are particularly effective with -diazo- -dicarbonyl
compounds such as diethyl diazomalonate.
284
F. Kido, S. C. Sinha, T. Abiko, M. Watanabe, and A. Yoshikoshi, Tetrahedron, 46, 4887 (1990).
285 C. J. Moody and R. J. Taylor, Tetrahedron, 46, 6501 (1990).
286 I. Coldham, M. L. Middleton, and P. L. Taylor, J. Chem. Soc., Perkin Trans. 1, 2951 (1997); I. Coldham,
M. L. Midleton, and P. L. Taylor, J. Chem. Soc., Perkin Trans. 1, 2817 (1998).
287
J. S. Clark and M. L. Middleton, Org. Lett., 4, 765 (2002).