Page 611 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 611
585
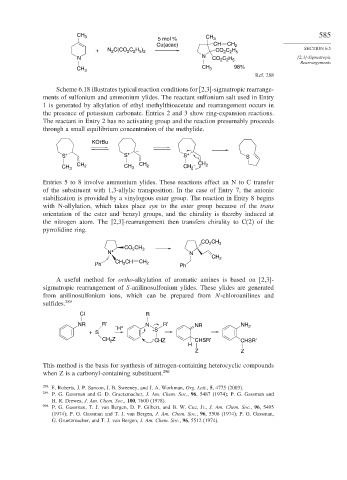
CH 3
5 mol % CH 3
Cu(acac) CH CH 2
+ N C(CO C H ) CO C H SECTION 6.5
2
2 2 5
2 2 5 2
N N CO 2 C 2 H 5 [2,3]-Sigmatropic
Rearrangements
CH 3 CH 3 98%
Ref. 288
Scheme 6.18 illustrates typical reaction conditions for [2,3]-sigmatropic rearrange-
ments of sulfonium and ammonium ylides. The reactant sulfonium salt used in Entry
1 is generated by alkylation of ethyl methylthioacetate and rearrangement occurs in
the presence of potassium carbonate. Entries 2 and 3 show ring-expansion reactions.
The reactant in Entry 2 has no activating group and the reaction presumably proceeds
through a small equilibrium concentration of the methylide.
KOt Bu
–
S + S + S + S
CH CH – CH 2
CH 3 2 CH 3 2 CH 2
Entries 5 to 8 involve ammonium ylides. These reactions effect an N to C transfer
of the substituent with 1,3-allylic transposition. In the case of Entry 7, the anionic
stabilization is provided by a vinylogous ester group. The reaction in Entry 8 begins
with N-allylation, which takes place syn to the ester group because of the trans
orientation of the ester and benzyl groups, and the chirality is thereby induced at
the nitrogen atom. The [2,3]-rearrangement then transfers chirality to C(2) of the
pyrrolidine ring.
CO CH 3
2
CO CH 3
2
N + N
CH 2
CH CH CH
Ph 2 2 Ph
A useful method for ortho-alkylation of aromatic amines is based on [2,3]-
sigmatropic rearrangement of S-anilinosulfonium ylides. These ylides are generated
from anilinosulfonium ions, which can be prepared from N-chloroanilines and
sulfides. 289
Cl R
NR R′ N R′ NR NH
– + 2
H
+ S +S
Z –
CH 2 CHZ CHSR′ CHSR′
H
Z Z
This method is the basis for synthesis of nitrogen-containing heterocyclic compounds
when Z is a carbonyl-containing substituent. 290
288
E. Roberts, J. P. Sancon, J. B. Sweeney, and J. A. Workman, Org. Lett., 5, 4775 (2003).
289 P. G. Gassman and G. D. Gruetzmacher, J. Am. Chem. Soc., 96, 5487 (1974); P. G. Gassman and
H. R. Drewes, J. Am. Chem. Soc., 100, 7600 (1978).
290
P. G. Gassman, T. J. van Bergen, D. P. Gilbert, and B. W. Cue, Jr., J. Am. Chem. Soc., 96, 5495
(1974); P. G. Gassman and T. J. van Bergen, J. Am. Chem. Soc., 96, 5508 (1974); P. G. Gassman,
G. Gruetzmacher, and T. J. van Bergen, J. Am. Chem. Soc., 96, 5512 (1974).