Page 616 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 616
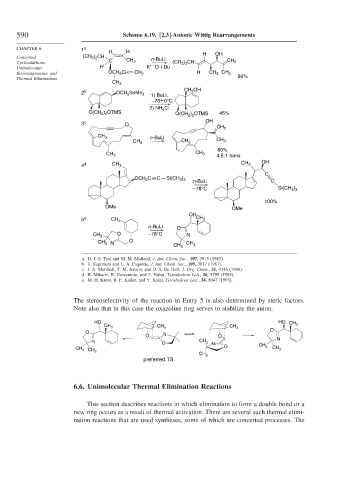
590 Scheme 6.19. [2,3]-Anionic Wittig Rearrangements
CHAPTER 6 1 a
H H H OH
Concerted (CH 3 ) 2 CH n -BuLi
Cycloadditions, C CH 3 (CH 3 ) 2 CH CH 2
+ –
Unimolecular H K O-t -Bu
Rearrangements, and OCH 2 CH CH 2 H CH 3 CH 3
Thermal Eliminations 95%
CH 3
CH 2 OH
2 b OCH 2 SnMe 3
1) BuLi,
–78 0°C
2) NH 4 Cl
O(CH 2 ) 2 OTMS O(CH 2 ) 2 OTMS 45%
3 c O OH
CH 2
CH 3
n -BuLi
CH 3 CH 3 CH 3
60%
CH 3
CH 3
4.5:1 trans
OH
4 d CH 3 CH 3
C
OCH 2 C C Si(CH 3 ) 3
n -BuLi C
–78°C Si(CH 3 ) 3
100%
OMe OMe
OH
5 e CH 3 CH 3
n -BuLi O
O –78°C
CH 3 N
O
CH 3 N CH 3
CH 3
a. D. J.-S. Tsai and M. M. Midland, J. Am. Chem. Soc., 107, 3915 (1985).
b. T. Sugimura and L. A. Paquette, J. Am. Chem. Soc., 109, 3017 (1987).
c. J. A. Marshall, T. M. Jenson, and D. S. De Hoff, J. Org. Chem., 51, 4316 (1986).
d. K. Mikami, K. Kawamoto, and T. Nakai, Tetrahedron Lett., 26, 5799 (1985).
e. M. H. Kress, B. F. Kaller, and Y. Kishi, Tetrahedron Lett., 34, 8047 (1993).
The stereoselectivity of the reaction in Entry 5 is also determined by steric factors.
Note also that in this case the oxazoline ring serves to stabilize the anion.
HO HO CH
CH 3 CH 3 CH 3 3
O O
O N O
N O CH 3 N N
CH 3 CH O CH 3 CH 3
3
CH 3
preferred TS
6.6. Unimolecular Thermal Elimination Reactions
This section describes reactions in which elimination to form a double bond or a
new ring occurs as a result of thermal activation. There are several such thermal elimi-
nation reactions that are used syntheses, some of which are concerted processes. The