Page 621 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 621
N N 595
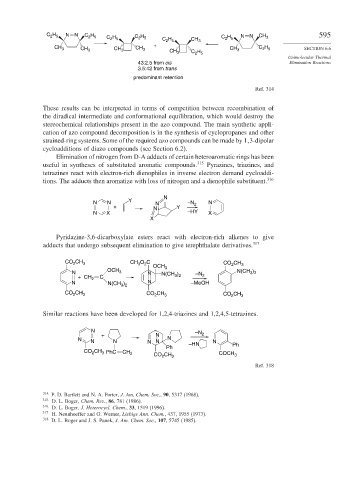
C 2 H 5 C 2 H 5 NN CH 3
C 2 H 5 C 2 H 5 C 2 H 5
C 2 H 5 CH 3
+
CH 3 CH 3 C 2 H 5 SECTION 6.6
CH 3 CH 3 CH 3
CH 3 C 2 H 5
Unimolecular Thermal
43:2.5 from cis Elimination Reactions
3.5:42 from trans
predominant retention
Ref. 314
These results can be interpreted in terms of competition between recombination of
the diradical intermediate and conformational equilibration, which would destroy the
stereochemical relationships present in the azo compound. The main synthetic appli-
cation of azo compound decomposition is in the synthesis of cyclopropanes and other
strained-ring systems. Some of the required azo compounds can be made by 1 3-dipolar
cycloadditions of diazo compounds (see Section 6.2).
Elimination of nitrogen from D-A adducts of certain heteroaromatic rings has been
useful in syntheses of substituted aromatic compounds. 315 Pyrazines, triazines, and
tetrazines react with electron-rich dienophiles in inverse electron demand cycloaddi-
tions. The adducts then aromatize with loss of nitrogen and a dienophile substituent. 316
N
Y
N N N –N 2 N
+ Y
N
N X –HY X
X
Pyridazine-3,6-dicarboxylate esters react with electron-rich alkenes to give
adducts that undergo subsequent elimination to give terephthalate derivatives. 317
CH
CO 2 3 CH O C OCH 3 CO CH 3
3
2
2
N OCH 3 N N(CH ) –N N(CH )
3 2
+ CH 2 C 3 2 2
N N(CH ) N –MeOH
3 2
CO CH 3 CO 2 CH 3 CO CH 3
2
2
Similar reactions have been developed for 1,2,4-triazines and 1,2,4,5-tetrazines.
N –N
+ N 2
N N N N N N N
Ph –HN Ph
CO CH PhC CH 2 CO 2 CH 3 COCH 3
3
2
Ref. 318
314 P. D. Bartlett and N. A. Porter, J. Am. Chem. Soc., 90, 5317 (1968).
315
D. L. Boger, Chem. Rev., 86, 781 (1986).
316
D. L. Boger, J. Heterocycl. Chem., 33, 1519 (1996).
317 H. Neunhoeffer and G. Werner, Liebigs Ann. Chem., 437, 1955 (1973).
318
D. L. Boger and J. S. Panek, J. Am. Chem. Soc., 107, 5745 (1985).