Page 622 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 622
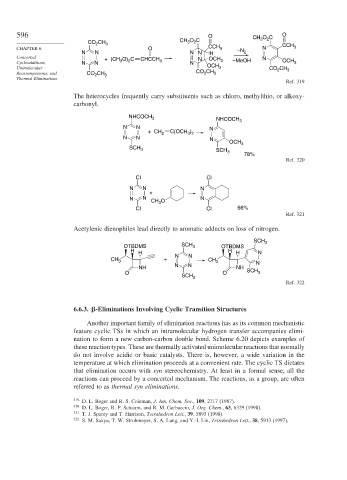
596 O CH O C O
CH CH 3 O C 3 2
2
CO 2 3 CCH
CHAPTER 6 O CCH 3 –N N 3
N N N N H 2
Concerted + (CH O) C CHCCH N OCH N
Cycloadditions, N N 3 2 3 N 3 –MeOH OCH 3
Unimolecular OCH 3 CO CH 3
2
Rearrangements, and CO CH 3 CO CH 3
2
2
Thermal Eliminations
Ref. 319
The heterocycles frequently carry substituents such as chloro, methylthio, or alkoxy-
carbonyl.
NHCOCH 3
NHCOCH 3
N N N
+ CH 2 C(OCH )
3 2
N N N
OCH 3
SCH 3 SCH
3
78%
Ref. 320
Cl Cl
N N N
+
N N CH O N
3
Cl Cl 66%
Ref. 321
Acetylenic dienophiles lead directly to aromatic adducts on loss of nitrogen.
SCH 3
OTBDMS SCH 3 OTBDMS
H H H H N
CH 3 + N N CH 3 N
NH N N NH
O O SCH 3
SCH 3
Ref. 322
6.6.3. -Eliminations Involving Cyclic Transition Structures
Another important family of elimination reactions has as its common mechanistic
feature cyclic TSs in which an intramolecular hydrogen transfer accompanies elimi-
nation to form a new carbon-carbon double bond. Scheme 6.20 depicts examples of
these reaction types. These are thermally activated unimolecular reactions that normally
do not involve acidic or basic catalysts. There is, however, a wide variation in the
temperature at which elimination proceeds at a convenient rate. The cyclic TS dictates
that elimination occurs with syn stereochemistry. At least in a formal sense, all the
reactions can proceed by a concerted mechanism. The reactions, as a group, are often
referred to as thermal syn eliminations.
319
D. L. Boger and R. S. Coleman, J. Am. Chem. Soc., 109, 2717 (1987).
320
D. L. Boger, R. P. Schaum, and R. M. Garbaccio, J. Org. Chem., 63, 6329 (1998).
321 T. J. Sparey and T. Harrison, Tetrahedron Lett., 39, 5893 (1998).
322
S. M. Sakya, T. W. Strohmeyer, S. A. Lang, and Y.-I. Lin, Tetrahedron Lett., 38, 5913 (1997).