Page 600 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 600
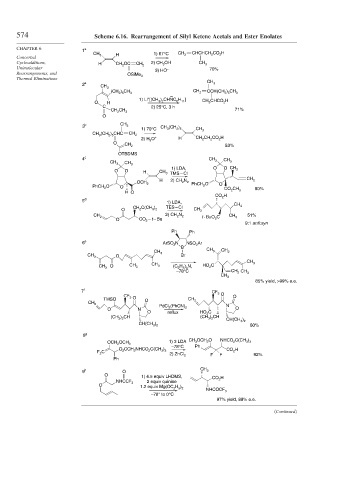
574 Scheme 6.16. Rearrangement of Silyl Ketene Acetals and Ester Enolates
CHAPTER 6
1 a
CH 3 H 1) 67°C CH 2 CHCHCH 2 CO 2 H
Concerted
Cycloadditions, H CH 2 OC CH 2 2) CH 3 OH CH 3
Unimolecular 3) HO – 70%
Rearrangements, and
OSiMe 3
Thermal Eliminations
2 a CH 3
CH 3
CH 2
(CH 2 ) 5 CH 3 CCH(CH 2 ) 5 CH 3
–
+
1) Li [(CH 3 ) 2 CHNC 6 H 11 ] CH 3 CHCO 2 H
O H
C 2) 25°C, 3 h
CH 2 CH 3 71%
O
3 b CH 3
1) 70°C CH 3 (CH 2 ) 5 CH 3
CH 3 (CH 2 ) 5 CHC CH 2
2) H 3 O + H CH 2 CH 2 CO 2 H
O
CH 2 53%
OTBDMS
4 c CH 3 CH 3
CH 3
CH 3
1) LDA, O O CH 3
O O H CH 3 TMS – Cl
H CH 2
OCH 2 2) CH 2 N 2 PhCH 2 O O
PhCH 2 O O
CO 2 CH 3 80%
H O
CO 2 H
d
5
1) LDA,
TES – Cl CH 3
O CH 2 C(CH 3 ) 2 CH 2
2) CH 2 N 2 51%
CH 2 t - BuO 2 C CH 3
O CO 2 – t – Bu
9:1 anti:syn
Ph Ph
6 e ArSO 2 N NSO 2 Ar
B
CH 3
CH 2
CH 3
CH 3 O Br
CH 3
O CH 3 HO 2 C
CH 2 CH 3 (C 2 H 5 ) N,
3
–78°C CH 2 CH 3
CH 3
85% yield, >99% e.e.
7 f
CF 3
O
CF 3 O O
TMSO O CH 2
CH 2
N
PdCl 2 (PhCN) 2
O N O
O reflux HO 2 C
(CH 3 ) 2 CH (CH 3 ) 2 CH
CH(CH 3 ) 2
CH(CH 3 ) 2 60%
8 g
1) 3 LDA CH 3 OCH 2 O NHCO 2 C(CH 3 ) 3
OCH 2 OCH 3
–78°C Ph
F 2 C O 2 CCH 2 NHCO 2 C(CH 3 ) 3 CO 2 H
2) ZnCl 2 F F 92%
Ph
9 h O CH 3
O 1) 4.5 equiv LHDMS, CO 2 H
NHCCF 3 2 equiv quinine
O
1.2 equiv Mg(OC 2 H 5 ) 2
NHCOCF 3
–78° to 0°C
97% yield, 88% e.e.
(Continued)