Page 599 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 599
Ar 573
H R
O SECTION 6.4
SO 2
N CH
Ph CH 3 [3,3]-Sigmatropic
B O 3
Ph Rearrangements
N
SO 2
Ar
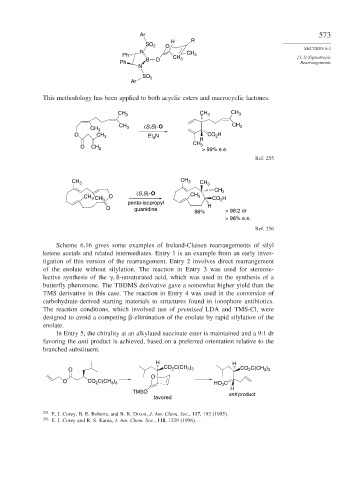
This methodology has been applied to both acyclic esters and macrocyclic lactones.
CH 3 CH 3 CH 3
CH CH 3
CH 3 3 (S,S)- O
O CH 3 Et N CO H
2
3
H
CH 3
O CH 2 > 99% e.e.
Ref. 255
CH 3 CH 3 CH 3
CH 2
(S,S)- O CH
CH 3 CH O 3 CO H
3
penta-isopropyl 2
O guanidine H
86% > 98:2 dr
> 98% e.e.
Ref. 256
Scheme 6.16 gives some examples of Ireland-Claisen rearrangements of silyl
ketene acetals and related intermediates. Entry 1 is an example from an early inves-
tigation of this version of the rearrangement. Entry 2 involves direct rearrangement
of the enolate without silylation. The reaction in Entry 3 was used for stereose-
lective synthesis of the
-unsaturated acid, which was used in the synthesis of a
butterfly pheromone. The TBDMS derivative gave a somewhat higher yield than the
TMS derivative in this case. The reaction in Entry 4 was used in the conversion of
carbohydrate-derived starting materials to structures found in ionophore antibiotics.
The reaction conditions, which involved use of premixed LDA and TMS-Cl, were
designed to avoid a competing -elimination of the enolate by rapid silylation of the
enolate.
In Entry 5, the chirality at an alkylated succinate ester is maintained and a 9:1 dr
favoring the anti product is achieved, based on a preferred orientation relative to the
branched substituent.
H H
CO C(CH )
O 2 3 3 CO C(CH )
2
3 3
O
O CO 2 C(CH ) HO 2 C
3 3
H
TMSO anti product
favored
255 E. J. Corey, B. E. Roberts, and B. R. Dixon, J. Am. Chem. Soc., 117, 193 (1995).
256
E. J. Corey and R. S. Kania, J. Am. Chem. Soc., 118, 1229 (1996).