Page 597 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 597
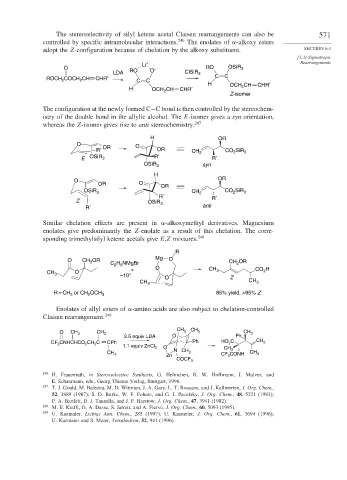
The stereoselectivity of silyl ketene acetal Claisen rearrangements can also be 571
controlled by specific intramolecular interactions. 246 The enolates of -alkoxy esters
adopt the Z-configuration because of chelation by the alkoxy substituent. SECTION 6.4
[3,3]-Sigmatropic
Li + Rearrangements
O – RO OSiR 3
LDA RO O ClSiR 3
COCH CH CHR′ C C
ROCH 2 2 C C H CHR′
2
H OCH CH CHR′ OCH CH
2
Z-isomer
The configuration at the newly formed C−C bond is then controlled by the stereochem-
istry of the double bond in the allylic alcohol. The E-isomer gives a syn orientation,
whereas the Z-isomer gives rise to anti stereochemistry. 247
H OR
O O
R′ OR OR CH CO SiR 3
2
R′ 2
E OSiR 3 R′
OSiR 3 syn
H
OR
O
OR O
OR
OSiR 3 CH 2 CO SiR 3
2
R′ R′
Z OSiR 3
R′ anti
Similar chelation effects are present in -alkoxymethyl derivatives. Magnesium
enolates give predominantly the Z-enolate as a result of this chelation. The corre-
sponding trimethylsilyl ketene acetals give E,Z mixtures. 248
R
O CH 2 OR Mg O CH OR
H NMgBr
C 2 5 2
O CH CO H
O 3 2
CH 3
–10° O Z
CH
CH 3 3
R = CH or CH OCH 3 85% yield, >95% Z
2
3
Enolates of allyl esters of -amino acids are also subject to chelation-controlled
Claisen rearrangement. 249
CH CH
O CH 3 CH 3 3 3 CH 3
2.5 equiv LDA O Ph
CF 3 CNHCHCO 2 CH 2 C CPh Ph HO C CH 2
2
O CH 3
1.1 equiv ZnCl 2
CH 3 Zn N CH 3 CF 3 CONH CH 3
COCF 3
246 H. Frauenrath, in Stereoselective Synthesis, G. Helmchen, R. W. Hoffmann, J. Mulzer, and
E. Schaumann, eds., Georg Thieme Verlag, Stuttgart, 1996.
247
T. J. Gould, M. Balestra, M. D. Wittman, J. A. Gary, L. T. Rossano, and J. Kallmerten, J. Org. Chem.,
52, 3889 (1987); S. D. Burke, W. F. Fobare, and G. J. Pacofsky, J. Org. Chem., 48, 5221 (1983);
P. A. Bartlett, D. J. Tanzella, and J. F. Barstow, J. Org. Chem., 47, 3941 (1982).
248 M. E. Krafft, O. A. Dasse, S. Jarrett, and A. Fierve, J. Org. Chem., 60, 5093 (1995).
249
U. Kazmaier, Liebigs Ann. Chem., 285 (1997); U. Kazmeier, J. Org. Chem., 61, 3694 (1996);
U. Kazmaier and S. Maier, Tetrahedron, 52, 941 (1996).