Page 594 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 594
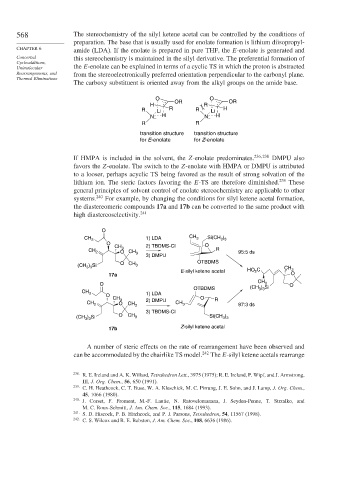
568 The stereochemistry of the silyl ketene acetal can be controlled by the conditions of
preparation. The base that is usually used for enolate formation is lithium diisopropyl-
CHAPTER 6
amide (LDA). If the enolate is prepared in pure THF, the E-enolate is generated and
Concerted this stereochemistry is maintained in the silyl derivative. The preferential formation of
Cycloadditions,
Unimolecular the E-enolate can be explained in terms of a cyclic TS in which the proton is abstracted
Rearrangements, and from the stereoelectronically preferred orientation perpendicular to the carbonyl plane.
Thermal Eliminations
The carboxy substituent is oriented away from the alkyl groups on the amide base.
O O
H OR R OR
R Li R R Li H
N – H N – H
R R
transition structure transition structure
for E-enolate for Z-enolate
If HMPA is included in the solvent, the Z-enolate predominates. 236 238 DMPU also
favors the Z-enolate. The switch to the Z-enolate with HMPA or DMPU is attributed
to a looser, perhaps acyclic TS being favored as the result of strong solvation of the
lithium ion. The steric factors favoring the E-TS are therefore diminished. 239 These
general principles of solvent control of enolate stereochemistry are applicable to other
systems. 240 For example, by changing the conditions for silyl ketene acetal formation,
the diastereomeric compounds 17a and 17b can be converted to the same product with
high diastereoselectivity. 241
O
1) LDA CH )
CH 3 3 Si(CH 3 3
O O
CH 3 2) TBDMS-Cl
CH 2 O CH R 95:5 ds
3
3) DMPU
O CH OTBDMS
(CH ) Si 3 CH
3 3
E-silyl ketene acetal HO 2 C 3
17a O
CH
O 3 O
OTBDMS (CH ) Si
3 3
CH 3 1) LDA
O
CH O R
CH 2 O 3 CH 3 2) DMPU CH 3 97:3 ds
3) TBDMS-Cl
(CH ) Si O CH 3 Si(CH )
3 3
3 3
17b Z-silyl ketene acetal
A number of steric effects on the rate of rearrangement have been observed and
can be accommodated by the chairlike TS model. 242 The E-silyl ketene acetals rearrange
238 R. E. Ireland and A. K. Willard, Tetrahedron Lett., 3975 (1975); R. E. Ireland, P. Wipf, and J. Armstrong,
III, J. Org. Chem., 56, 650 (1991).
239 C. H. Heathcock, C. T. Buse, W. A. Kleschick, M. C. Pirrung, J. E. Sohn, and J. Lamp, J. Org. Chem.,
45, 1066 (1980).
240
J. Corset, F. Froment, M.-F. Lautie, N. Ratovelomanana, J. Seyden-Penne, T. Strzalko, and
M. C. Roux-Schmitt, J. Am. Chem. Soc., 115, 1684 (1993).
241 S. D. Hiscock, P. B. Hitchcock, and P. J. Parsons, Tetrahedron, 54, 11567 (1998).
242
C. S. Wilcox and R. E. Babston, J. Am. Chem. Soc., 108, 6636 (1986).