Page 590 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 590
564 CH 2 OCH 3
CH 3 OH CH 3 O OCH
CHAPTER 6 CH 3 3
CH 3 CH POCl 3 CH CH CH 3 CH 3
Concerted 3 3 3
Cycloadditions,
Unimolecular CH CH 2 CH 3 O CH 3
Rearrangements, and 3 CH 3 R 3 Al
Thermal Eliminations O + CH 3 CH CH
–
CH CH Al R 3 2
3 3 OH 3
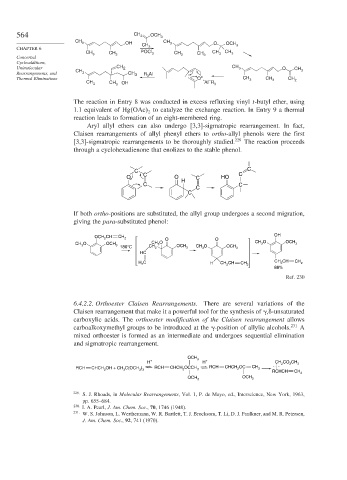
The reaction in Entry 8 was conducted in excess refluxing vinyl t-butyl ether, using
1.1 equivalent of Hg OAc to catalyze the exchange reaction. In Entry 9 a thermal
2
reaction leads to formation of an eight-membered ring.
Aryl allyl ethers can also undergo [3,3]-sigmatropic rearrangement. In fact,
Claisen rearrangements of allyl phenyl ethers to ortho-allyl phenols were the first
[3,3]-sigmatropic rearrangements to be thoroughly studied. 229 The reaction proceeds
through a cyclohexadienone that enolizes to the stable phenol.
C C
O C O C HO C
H
C C
C
C
If both ortho-positions are substituted, the allyl group undergoes a second migration,
giving the para-substituted phenol:
OCH CH CH 2 OH
2 O O
3
O OCH CH O CH O OCH
CH 3 3 3
3 CH OCH CH O OCH
180°C 2 3 3 3
HC
C CH CH CH 2
H 2 H CH 2 CH CH 2 2
88%
Ref. 230
6.4.2.2. Orthoester Claisen Rearrangements. There are several variations of the
Claisen rearrangement that make it a powerful tool for the synthesis of ,
-unsaturated
carboxylic acids. The orthoester modification of the Claisen rearrangement allows
carboalkoxymethyl groups to be introduced at the -position of allylic alcohols. 231 A
mixed orthoester is formed as an intermediate and undergoes sequential elimination
and sigmatropic rearrangement.
OCH
+ 3 +
H H CH CO CH
2 2 3
RCH CHCH 2 OH + CH C(OCH ) RCH CHCH 2 OCCH RCH CHCH 2 OC CH 2
3 3 3 3
RCHCH CH 2
OCH OCH
3 3
229
S. J. Rhoads, in Molecular Rearrangements, Vol. 1, P. de Mayo, ed., Interscience, New York, 1963,
pp. 655–684.
230 I. A. Pearl, J. Am. Chem. Soc., 70, 1746 (1948).
231
W. S. Johnson, L. Werthemann, W. R. Bartlett, T. J. Brocksom, T. Li, D. J. Faulkner, and M. R. Petersen,
J. Am. Chem. Soc., 92, 741 (1970).