Page 585 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 585
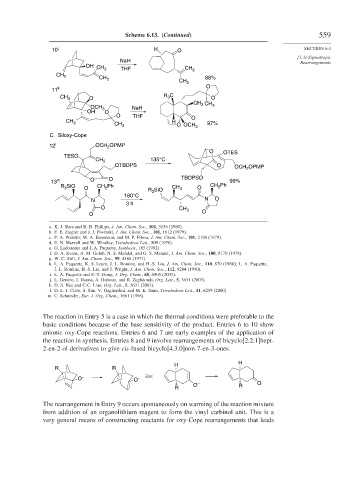
Scheme 6.13. (Continued) 559
j H SECTION 6.4
10 O
[3,3]-Sigmatropic
NaH Rearrangements
OH CH 3 THF CH 2
CH 2
CH 2 CH 88%
3
O
11 k
CH 3 O H C O
3
CH 3 CH 3
OCH 3 NaH
OH O
O THF O
CH 3 CH 3 H O OCH 3 97%
C. Siloxy-Cope
12 l OCH OPMP
2
O OTES
TESO
CH 2 135°C
OTBDPS O OCH OPMP
2
13 m O O TBDPSO 99%
R SiO O CH Ph R SiO CH 3 O CH 2 Ph
2
3
180°C 3
N N O
3 h
O CH 3
O O
a. K. J. Shea and R. B. Phillips, J. Am. Chem. Soc., 102, 3156 (1980).
b. F. E. Ziegler and J. J. Piwinski, J. Am. Chem. Soc., 101, 1612 (1979).
c. P. A. Wender, M. A. Eissenstat, and M. P. Filosa, J. Am. Chem. Soc., 101, 2196 (1979).
d. E. N. Marvell and W. Whalley, Tetrahedron Lett., 509 (1970).
e. G. Ladouceur and L.A. Paquette, Synthesis, 185 (1992)
f. D. A. Evans, A. M. Golob, N. S. Mandel, and G. S. Mandel, J. Am. Chem. Soc., 100, 8170 (1978).
g. W. C. Still, J. Am. Chem. Soc., 99, 4186 (1977).
h. L. A. Paquette, K. S. Learn, J. L. Romine, and H.-S. Lin, J. Am. Chem. Soc., 110, 879 (1988); L. A. Paquette,
J. L. Romine, H.-S. Lin, and J. Wright, J. Am. Chem. Soc., 112, 9284 (1990).
i. L. A. Paquette and F.-T. Hong, J. Org. Chem., 68, 6905 (2003).
j. L. Gentric, I. Hanna, A. Huboux, and R. Zaghdoudi, Org. Lett., 5, 3631 (2003).
k. D. S. Hsu and C-C. Liao, Org. Lett., 5, 3631 (2003).
l. D. L. J. Clive, S. Sun, V. Gagliardini, and M. K. Sano, Tetrahedron Lett., 41, 6259 (2000).
m. C. Schneider, Eur. J. Org. Chem., 1661 (1998).
The reaction in Entry 5 is a case in which the thermal conditions were preferable to the
basic conditions because of the base sensitivity of the product. Entries 6 to 10 show
anionic oxy-Cope reactions. Entries 6 and 7 are early examples of the application of
the reaction in synthesis. Entries 8 and 9 involve rearrangements of bicyclo[2.2.1]hept-
2-en-2-ol derivatives to give cis-fused bicyclo[4.3.0]non-7-en-3-ones.
H H
R R
O – O –
O – R O
R
The rearrangement in Entry 9 occurs spontaneously on warming of the reaction mixture
from addition of an organolithium reagent to form the vinyl carbinol unit. This is a
very general means of constructing reactants for oxy-Cope rearrangements that leads