Page 583 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 583
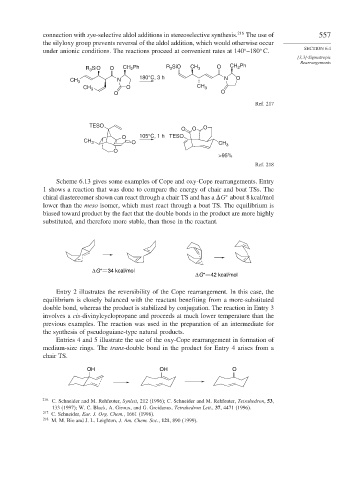
connection with syn-selective aldol additions in stereoselective synthesis. 216 The use of 557
the silyloxy group prevents reversal of the aldol addition, which would otherwise occur
SECTION 6.4
under anionic conditions. The reactions proceed at convenient rates at 140 –180 C.
[3,3]-Sigmatropic
Rearrangements
R 3 SiO O CH 2 Ph R 3 SiO CH 3 O CH 2 Ph
180°C, 3 h N O
CH 3 N
CH 3 O CH 3
O O
Ref. 217
TESO
O O O
O 105°C, 1 h TESO
CH 3 O CH 3
O
>95%
Ref. 218
Scheme 6.13 gives some examples of Cope and oxy-Cope rearrangements. Entry
1 shows a reaction that was done to compare the energy of chair and boat TSs. The
chiral diastereomer shown can react through a chair TS and has a
G about 8 kcal/mol
∗
lower than the meso isomer, which must react through a boat TS. The equilibrium is
biased toward product by the fact that the double bonds in the product are more highly
substituted, and therefore more stable, than those in the reactant.
ΔG* 34 kcal/mol
ΔG* 42 kcal/mol
Entry 2 illustrates the reversibility of the Cope rearrangement. In this case, the
equilibrium is closely balanced with the reactant benefiting from a more-substituted
double bond, whereas the product is stabilized by conjugation. The reaction in Entry 3
involves a cis-divinylcyclopropane and proceeds at much lower temperature than the
previous examples. The reaction was used in the preparation of an intermediate for
the synthesis of pseudoguiane-type natural products.
Entries 4 and 5 illustrate the use of the oxy-Cope rearrangement in formation of
medium-size rings. The trans-double bond in the product for Entry 4 arises from a
chair TS.
OH OH O
216
C. Schneider and M. Rehfeuter, Synlett, 212 (1996); C. Schneider and M. Rehfeuter, Tetrahedron, 53,
133 (1997); W. C. Black, A. Giroux, and G. Greidanus, Tetrahedron Lett., 37, 4471 (1996).
217 C. Schneider, Eur. J. Org. Chem., 1661 (1998).
218
M. M. Bio and J. L. Leighton, J. Am. Chem. Soc., 121, 890 (1999).