Page 586 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 586
560 to carbon-carbon bond formation between C(2) of the vinyllithium reagent and C(4)
of the , -enone.
CHAPTER 6
Concerted OLi
Cycloadditions, O OLi R 4 O R 2
Unimolecular R 4 R 4 R
Rearrangements, and R R R
Thermal Eliminations 4
+ LiCH CHR 2 R 2 R
R 2
The reaction in Entry 10 demonstrated that a vinyl substituent in conjugation with the
vinyl carbinol accelerates rearrangement. The reaction was considerably more facile
than the corresponding reaction with a saturated isopropyl group. The reaction in Entry
11 was used in the synthesis of terpene derivatives. Entries 12 and 13 are examples
of the siloxy-Cope version of the reaction. These entries illustrate the utility of the
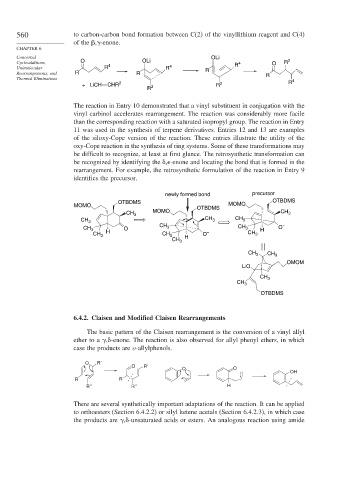
oxy-Cope reaction in the synthesis of ring systems. Some of these transformations may
be difficult to recognize, at least at first glance. The retrosynthetic transformation can
be recognized by identifying the
, -enone and locating the bond that is formed in the
rearrangement. For example, the retrosynthetic formulation of the reaction in Entry 9
identifies the precursor.
newly formed bond precursor
OTBDMS MOMO OTBDMS
MOMO OTBDMS
CH 3 MOMO CH 3
CH 3 CH 3 CH 3
CH CH –
CH 3 O 3 3 H O
CH 3 H CH 3 O – CH 3
CH 3 H
CH 3 CH 3
OMOM
LiO
CH 3
CH 3
OTBDMS
6.4.2. Claisen and Modified Claisen Rearrangements
The basic pattern of the Claisen rearrangement is the conversion of a vinyl allyl
ether to a ,
-enone. The reaction is also observed for allyl phenyl ethers, in which
case the products are o-allylphenols.
O R′
O R′
O O
OH
R R
R′′ R′′ H
There are several synthetically important adaptations of the reaction. It can be applied
to orthoesters (Section 6.4.2.2) or silyl ketene acetals (Section 6.4.2.3), in which case
the products are ,
-unsaturated acids or esters. An analogous reaction using amide