Page 584 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 584
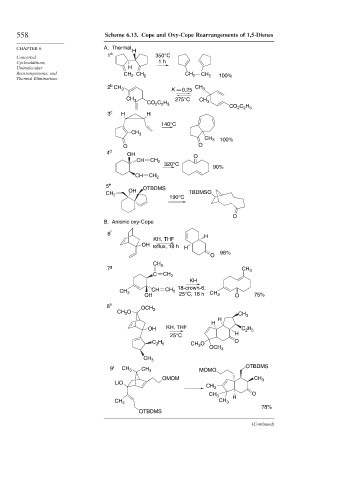
558 Scheme 6.13. Cope and Oxy-Cope Rearrangements of 1,5-Dienes
CHAPTER 6 A. Thermal H
1 a 350°C
Concerted
Cycloadditions, 1 h
Unimolecular H
Rearrangements, and CH CH 2 CH 2 CH 100%
2
Thermal Eliminations 2
2 b CH 3 K 0.25 CH 3
CH 3 275°C CH
CO C H 3
2 2 5
CO C H
2 2 5
3 c H H
140°C
CH 3
CH 3 100%
O O
4 d OH O
CH CH 2
320°C
90%
CH CH 2
5 e OTBDMS
OH
CH 2 TBDMSO
190°C
O
B. Anionic oxy-Cope
6 f H
KH, THF
OH reflux, 18 h H
98%
O
CH 3
7 g CH 3
C CH 2
KH
CH 3 CH CH 2 18-crown-6,
OH 25°C, 18 h CH 3 O 75%
8 h OCH
CH O 3 CH
3
H 3
H
OH KH, THF C H
2 5
25°C H
C H O O
2 5
CH 3
OCH 3
CH 3
9 i CH 3 CH 3 MOMO OTBDMS
OMOM CH 3
LiO
CH 3
CH 3 O
CH 3 CH 3 H
78%
OTBDMS
(Continued)