Page 598 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 598
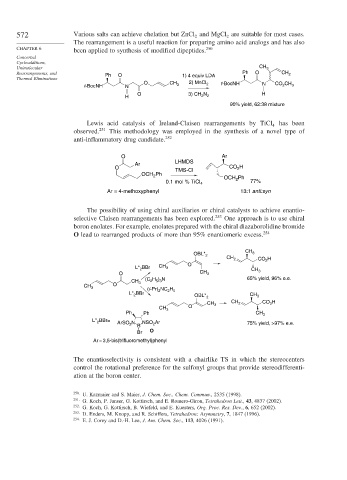
572 Various salts can achieve chelation but ZnCl and MgCl are suitable for most cases.
2
2
The rearrangement is a useful reaction for preparing amino acid analogs and has also
CHAPTER 6 250
been applied to synthesis of modified dipeptides.
Concerted
Cycloadditions,
Unimolecular CH 3
Rearrangements, and Ph O 1) 4 equiv LDA Ph O CH 2
Thermal Eliminations
O 2) MnCl 2 t-BocNH N CO CH
t-BocNH N CH 3 2 3
O 3) CH N H
H 2 2
90% yield, 62:38 mixture
Lewis acid catalysis of Ireland-Claisen rearrangements by TiCl 4 has been
observed. 251 This methodology was employed in the synthesis of a novel type of
anti-inflammatory drug candidate. 252
O Ar
LHMDS
Ar
O TMS-Cl CO H
2
Ph
OCH 2
OCH Ph
0.1 mol % TiCl 4 2 77%
Ar = 4-methoxyphenyl 13:1 anti:syn
The possibility of using chiral auxiliaries or chiral catalysts to achieve enantio-
selective Claisen rearrangements has been explored. 253 One approach is to use chiral
boron enolates. For example, enolates prepared with the chiral diazaborolidine bromide
O lead to rearranged products of more than 95% enantiomeric excess. 254
CH
OBL* 2 3
CH 2 CO 2 H
L* BBr CH 3 O CH
2
O CH 3 3
H ) N 65% yield, 96% e.e.
(C 2 5 3
CH 3
CH 3 O
2 5
(i-Pr) 2 NC H
L* BBr OBL* 2 CH 3
2
CH 3 CH 2 CO H
2
CH 3 O
Ph Ph CH 3
L* BBr=
2
2
ArSO 2 N NSO Ar 75% yield, >97% e.e.
B
Br O
Ar = 3,5-bis(trifluoromethyl)phenyl
The enantioselectivity is consistent with a chairlike TS in which the stereocenters
control the rotational preference for the sulfonyl groups that provide stereodifferenti-
ation at the boron center.
250
U. Kazmaier and S. Maier, J. Chem. Soc., Chem. Commun., 2535 (1998).
251
G. Koch, P. Janser, G. Kottirsch, and E. Romero-Giron, Tetrahedron Lett., 43, 4837 (2002).
252 G. Koch, G. Kottirsch, B. Wiefeld, and E. Kuesters, Org. Proc. Res. Dev., 6, 652 (2002).
253 D. Enders, M. Knopp, and R. Schiffers, Tetrahedron: Asymmetry, 7, 1847 (1996).
254
E. J. Corey and D.-H. Lee, J. Am. Chem. Soc., 113, 4026 (1991).