Page 601 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 601
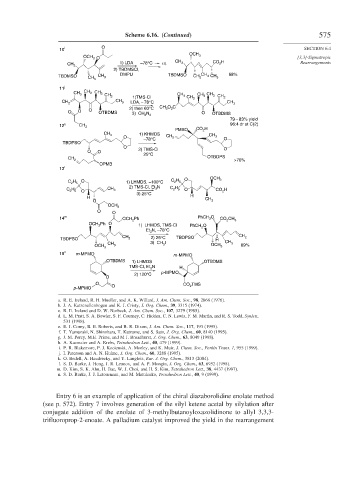
Scheme 6.16. (Continued) 575
10 i O SECTION 6.4
OCH 3
OCH 3 O [3,3]-Sigmatropic
1) LDA –78°C r.t. CH 3 CO 2 H Rearrangements
CH 3
2) TBDMSCl,
DMPU TBDMSO 68%
TBDMSO CH 3 CH 3 CH 3 CH 3
CH 3
11 j
CH 3
CH 3 CH 3
CH 3
CH 2 CH 3 CH 3 CH 2
1)TMS-Cl CH 3
CH 3
CH 2 LDA, – 78°C CH 3
2) then 60°C CH 3 O 2 C
O O O OTBDMS O
3) CH 2 N 2 OTBDMS
79 – 83% yield
12 k CH 3 96:4 dr at C(2)
PMBO CO 2 H
CH 3 1) KHMDS CH 3
O CH 2
–78°C O
TBDPSO
O 2) TMS-Cl O
O O
25°C
OTBDPS
>70%
CH 2
OPMB
13 l
O OCH 3
O C 2 H 5
C 2 H 5 1) LHMDS, –100°C
2) TMS-Cl, Et 3 N
C 2 H 5 CH 3 C 2 H 5 O CO 2 H
O
3) 25°C
H H
O CH 3
OCH 3
O O
14 m OCH 2 Ph PhCH 2 O CO 2 CH 3
OCH 2 Ph O 1) LHMDS, TMS-Cl PhCH 2 O
Et 3 N, –78°C
TBDPSO CH 2 2) 25°C TBDPSO H CH 2
3) CH 3 I CH 3
CH 3 89%
OCH 3
OCH 3
15 n m-MPMO m -MPMO
OTBDMS 1) LHMDS OTBDMS
TMS-Cl, Et 3 N H
p -MPMO
2) 120°C
O
O CO 2 TMS
p -MPMO O
a. R. E. Ireland, R. H. Mueller, and A. K. Willard, J. Am. Chem. Soc., 98, 2868 (1976).
b. J. A. Katzenellenbogen and K. J. Cristy, J. Org. Chem., 39, 3315 (1974).
c. R. E. Ireland and D. W. Norbeck, J. Am. Chem. Soc., 107, 3279 (1985).
d. L. M. Pratt, S. A. Bowler, S. F. Courney, C. Hidden, C. N. Lewis, F. M. Martin, and R. S. Todd, Synlett,
531 (1998).
e. E. J. Corey, B. E. Roberts, and B. R. Dixon, J. Am. Chem. Soc., 117, 193 (1995).
f. T. Yamazaki, N. Shinohara, T. Katzume, and S. Sato, J. Org. Chem., 60, 8140 (1995).
g. J. M. Percy, M.E. Prime, and M J. Broadhurst, J. Org. Chem., 63, 8049 (1998).
h. A. Kazmaier and A. Krebs, Tetrahedron Lett., 40, 479 (1999).
i. P. R. Blakemore, P. J. Kocienski, A. Morley, and K. Muir, J. Chem. Soc., Perkin Trans. 1, 955 (1999).
j. I. Paterson and A. N. Hulme, J. Org. Chem., 60, 3288 (1995).
k. O. Bedell, A. Haudrecky, and Y. Langlois, Eur. J. Org. Chem., 3813 (2004).
l. S. D. Burke, J. Hong, J. R. Lennox, and A. P. Mongin, J. Org. Chem., 63, 6952 (1998).
m. D. Kim, S. K. Ahn, H. Bae, W. J. Choi, and H. S. Kim, Tetrahedron Lett., 38, 4437 (1997).
n. S. D. Burke, J. J. Letourneau, and M. Matulenko, Tetrahedron Lett., 40, 9 (1999).
Entry 6 is an example of application of the chiral diazaborolidine enolate method
(see p. 572). Entry 7 involves generation of the silyl ketene acetal by silylation after
conjugate addition of the enolate of 3-methylbutanoyloxazolidinone to allyl 3,3,3-
trifluoroprop-2-enoate. A palladium catalyst improved the yield in the rearrangement