Page 596 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 596
570 The reason for the trend toward boat TSs in cyclic systems is the introduction of
additional steric factors. For example, addition of methyl and isopropenyl substituents
CHAPTER 6 leads to a TS in which the cyclohexene ring adopts a boat conformation, whereas the
Concerted TS is chairlike.
Cycloadditions,
Unimolecular
Rearrangements, and O TBDMS
Thermal Eliminations CH O CH CH 3
3
O CH 3
LDA CH 3 3 CH
2
CH TBDMSCl O CH HO C 3
3 2
CH 3
CH 3 45% DMPU O CH 3 CH 2
CH OTBS
3 CH
CH 3
2
CH
2
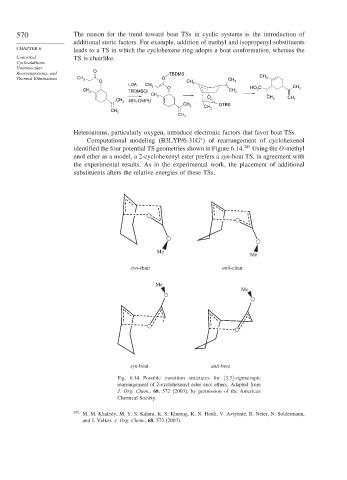
Heteroatoms, particularly oxygen, introduce electronic factors that favor boat TSs.
∗
Computational modeling (B3LYP/6-31G ) of rearrangement of cyclohexenol
identified the four potential TS geometries shown in Figure 6.14. 245 Using the O-methyl
enol ether as a model, a 2-cyclohexenyl ester prefers a syn-boat TS, in agreement with
the experimental results. As in the experimental work, the placement of additional
substituents alters the relative energies of these TSs.
Me
Me
syn-chair anti-chair
Me
Me
syn-boat anti-boat
Fig. 6.14. Possible transition structures for [3,3]-sigmatropic
rearrangement of 2-cyclohexenyl ester enol ethers. Adapted from
J. Org. Chem., 68, 572 (2003), by permission of the American
Chemical Society.
245
M. M. Khaledy, M. Y. S. Kalani, K. S. Khuong, K. N. Houk, V. Aviyente, R. Neier, N. Soldermann,
and J. Velker, J. Org. Chem., 68, 572 (2003).