Page 744 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 744
720 involvement of a cationic, as opposed to a neutral, complex. The triflate anion is more
likely to be dissociated than a halide.
CHAPTER 8
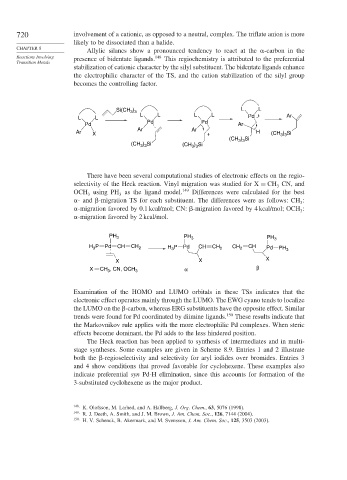
Allylic silanes show a pronounced tendency to react at the -carbon in the
Reactions Involving presence of bidentate ligands. 148 This regiochemistry is attributed to the preferential
Transition Metals
stabilization of cationic character by the silyl substituent. The bidentate ligands enhance
the electrophilic character of the TS, and the cation stabilization of the silyl group
becomes the controlling factor.
Si(CH ) L L
3 3
L L L L L L Pd Ar
Pd Pd Pd Ar
Ar Ar
Ar X + H (CH ) Si
3 3
(CH ) Si
3 3
(CH ) Si (CH 3 3
) Si
3 3
There have been several computational studies of electronic effects on the regio-
selectivity of the Heck reaction. Vinyl migration was studied for X = CH CN, and
3
OCH using PH as the ligand model. 149 Differences were calculated for the best
3 3
- and -migration TS for each substituent. The differences were as follows: CH :
3
-migration favored by 0.1 kcal/mol; CN: -migration favored by 4 kcal/mol; OCH :
3
-migration favored by 2 kcal/mol.
PH 3 PH 3 PH 3
H P Pd CH CH 2 H P Pd CH CH 2 CH 2 CH Pd PH 3
3
3
X X X
X CH , CN, OCH 3 α β
3
Examination of the HOMO and LUMO orbitals in these TSs indicates that the
electronic effect operates mainly through the LUMO. The EWG cyano tends to localize
the LUMO on the -carbon, whereas ERG substituents have the opposite effect. Similar
trends were found for Pd coordinated by diimine ligands. 150 These results indicate that
the Markovnikov rule applies with the more electrophilic Pd complexes. When steric
effects become dominant, the Pd adds to the less hindered position.
The Heck reaction has been applied to synthesis of intermediates and in multi-
stage syntheses. Some examples are given in Scheme 8.9. Entries 1 and 2 illustrate
both the -regioselectivity and selectivity for aryl iodides over bromides. Entries 3
and 4 show conditions that proved favorable for cyclohexene. These examples also
indicate preferential syn Pd-H elimination, since this accounts for formation of the
3-substituted cyclohexene as the major product.
148 K. Olofsson, M. Larhed, and A. Hallberg, J. Org. Chem., 63, 5076 (1998).
149 R. J. Deeth, A. Smith, and J. M. Brown, J. Am. Chem. Soc., 126, 7144 (2004).
150
H. V. Schenck, B. Akermark, and M. Svensson, J. Am. Chem. Soc., 125, 3503 (2003).