Page 745 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 745
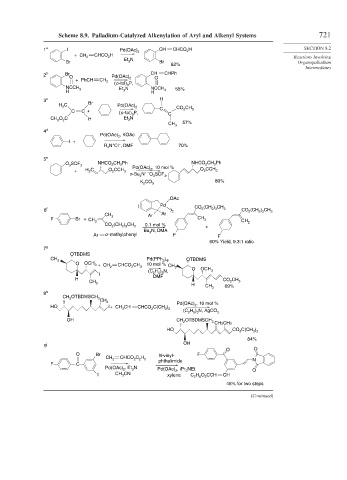
Scheme 8.9. Palladium-Catalyzed Alkenylation of Aryl and Alkenyl Systems 721
1 a I Pd(OAc) 2 CH CHCO 2 H SECTION 8.2
+ CH CHCO H
2 2 Reactions Involving
Br Et N Br Organopalladium
3
82%
Intermediates
2 b Br CH CHPh
O Pd(OAc) 2 O
+ PhCH CH 2
(o -tol) P,
3
NCCH 3 Et 3 N NCCH 55%
H H 3
3 c H
H C Br Pd(OAc) 2 CO CH
3
C C + (o -tol) P, C 2 3
3 C
O C N
CH 3 2 H Et 3
57%
CH 3
4 d
Pd(OAc) , KOAc
2
I +
+
–
R N Cl , DMF 70%
4
5 e
O SCF 3 NHCO CH Ph , 10 mol % NHCO CH Ph
2
2
3
2
3
+ H C O 2 CCH 3 Pd(OAc) 2 O CCH 3
2
2
3 3
n-Bu 4 N + – O SCF ,
K CO 3 80%
2
OAc
( Pd (CH ) CH
6 f ) 2 CO 2 2 3 3 CO (CH ) CH 3
2
2 3
CH Ar Ar
F Br + CH 2 3 CH 3 CH
CO (CH ) CH 3 0.1 mol % + 2
2 3
2
Bu N, DMA
3
Ar o -methylphenyl F F
60% Yield, 9.3:1 ratio
7 g
OTBDMS
CH Pd(PPh ) , OTBDMS
3 3 4
O OCH 3 + CH CHCO CH 10 mol %
2 2 3 CH 3 O OCH
H ) N, 3
(C 2 5 3
I DMF
H CO CH
CH 3 H 2 3
CH 3 69%
8 h
OTBDMSCH
CH 2
3
CH 3 Pd(OAc) , 10 mol %
HO I+ CH CH CHCO C(CH ) 2
3 2 3 3 H ) N, AgCO
(C 2 5 3 3
OH CH 2 OTBDMSCH
3
CH3CH3
HO CO C(CH )
3 3
2
84%
9 i OH
O O
O Br N-vinyl- F
CH CHCO C H
2 2 2 5 phthalimide N
F C
Pd(OAc) , Et N Pd(OAc) 2 , i Pr NEt O
3
2
I CH CN xylene 2 C H O CCH CH
3
5
2
2
46% for two steps
(Continued)