Page 748 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 748
724 Ligands and anions play a crucial role in determining the rates and equilibria of the
various steps by controlling the detailed coordination environment at palladium. 153
CHAPTER 8 In the next section we discuss coupling reactions involving organolithium, organo-
Reactions Involving magnesium, organozinc, and organocopper reagents. We then proceed to arylation of
Transition Metals
enolates mediated by palladium catalysts. Subsequent sections consider cross coupling
with stannanes (Stille reaction) and boron compounds (Suzuki reaction).
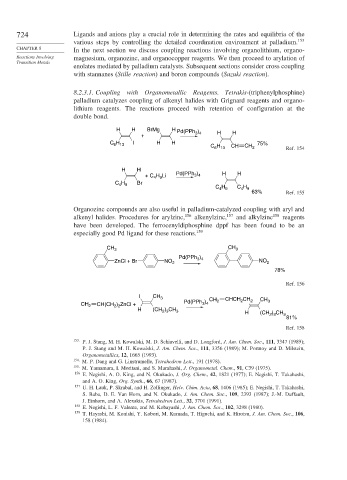
8.2.3.1. Coupling with Organometallic Reagents. Tetrakis-(triphenylphosphine)
palladium catalyzes coupling of alkenyl halides with Grignard reagents and organo-
lithium reagents. The reactions proceed with retention of configuration at the
double bond.
H H BrMg H Pd(PPh )
+ 3 4 H H
C H I H H 75%
6 13
H
C 6 13 CH CH 2 Ref. 154
H H
3 4
+ C H Li Pd(PPh ) H H
4 9
C H Br
4 9
C H C H
4 9
4 9
63% Ref. 155
Organozinc compounds are also useful in palladium-catalyzed coupling with aryl and
alkenyl halides. Procedures for arylzinc, 156 alkenylzinc, 157 and alkylzinc 158 reagents
have been developed. The ferrocenyldiphosphine dppf has been found to be an
especially good Pd ligand for these reactions. 159
CH
CH 3 3
Pd(PPh )
ZnCl + Br NO 2 3 4 NO 2
78%
Ref. 156
I CH 3 CH CHCH CH CH
3 4
CH 2 CH(CH ) ZnCl + Pd(PPh ) 2 2 2 3
2 2
H (CH ) CH 3 H (CH ) CH
2 3
2 3
3
81%
Ref. 158
153
P. J. Stang, M. H. Kowalski, M. D. Schiavelli, and D. Longford, J. Am. Chem. Soc., 111, 3347 (1989);
P. J. Stang and M. H. Kowalski, J. Am. Chem. Soc., 111, 3356 (1989); M. Portnoy and D. Milstein,
Organometallics, 12, 1665 (1993).
154 M. P. Dang and G. Linstrumelle, Tetrahedron Lett., 191 (1978).
155 M. Yamamura, I. Moritani, and S. Murahashi, J. Organometal. Chem., 91, C39 (1975).
156
E. Negishi, A. O. King, and N. Okukado, J. Org. Chem., 42, 1821 (1977); E. Negishi, T. Takahashi,
and A. O. King, Org. Synth., 66, 67 (1987).
157 U. H. Lauk, P. Skrabal, and H. Zollinger, Helv. Chim. Acta, 68, 1406 (1985); E. Negishi, T. Takahashi,
S. Baba, D. E. Van Horn, and N. Okukado, J. Am. Chem. Soc., 109, 2393 (1987); J.-M. Duffault,
J. Einhorn, and A. Alexakis, Tetrahedron Lett., 32, 3701 (1991).
158 E. Negishi, L. F. Valente, and M. Kobayashi, J. Am. Chem. Soc., 102, 3298 (1980).
159
T. Hayashi, M. Konishi, Y. Kobori, M. Kumada, T. Higuchi, and K. Hirotsu, J. Am. Chem. Soc., 106,
158 (1984).