Page 752 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 752
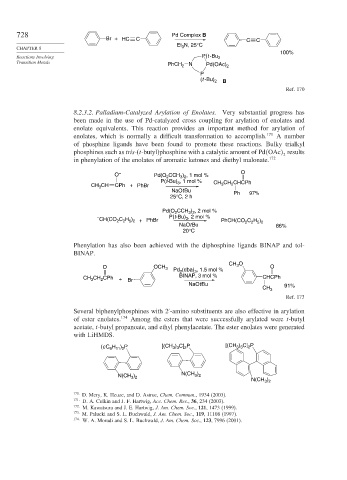
728 Pd Complex B
Br + HC C C C
Et N, 25°C
CHAPTER 8 3
100%
Reactions Involving P(t -Bu 2
Transition Metals PhCH 2 N Pd(OAc) 2
P
(t -Bu) 2 B
Ref. 170
8.2.3.2. Palladium-Catalyzed Arylation of Enolates. Very substantial progress has
been made in the use of Pd-catalyzed cross coupling for arylation of enolates and
enolate equivalents. This reaction provides an important method for arylation of
enolates, which is normally a difficult transformation to accomplish. 171 A number
of phosphine ligands have been found to promote these reactions. Bulky trialkyl
phosphines such as tris-(t-butyl)phosphine with a catalytic amount of Pd OAc results
2
in phenylation of the enolates of aromatic ketones and diethyl malonate. 172
O – Pd(O CCH ) , 1 mol % O
2
3 2
P(t-Bu) , 1 mol % CH CHCPh
3
CH CH CPh + PhBr CH 3 2
3
NaOt Bu Ph 97%
25°C, 2 h
Pd(O CCH ) , 2 mol %
3 2
2
– CH(CO C H ) PhBr P(t-Bu) , 2 mol % C H )
3
2 2 5 2 +
NaOt Bu PhCH(CO 2 2 5 2 86%
20°C
Phenylation has also been achieved with the diphosphine ligands BINAP and tol-
BINAP.
CH O
O OCH 3 3 O
Pd (dba) , 1.5 mol %
2
3
BINAP, 3 mol %
CH CH CPh + Br CHCPh
2
3
NaOt Bu 91%
CH 3
Ref. 173
Several biphenylphosphines with 2 -amino substituents are also effective in arylation
of ester enolates. 174 Among the esters that were successfully arylated were t-butyl
acetate, t-butyl propanoate, and ethyl phenylacetate. The ester enolates were generated
with LiHMDS.
(c C H ) P [(CH ) C] P [(CH ) C] P
2
3 3
3 3
2
6 11 2
N(CH ) N(CH )
3 2
3 2
N(CH )
3 2
170 D. Mery, K. Heuze, and D. Astruc, Chem. Commun., 1934 (2003).
171
D. A. Culkin and J. F. Hartwig, Acc. Chem. Res., 36, 234 (2003).
172
M. Kawatsura and J. E. Hartwig, J. Am. Chem. Soc., 121, 1473 (1999).
173 M. Palucki and S. L. Buchwald, J. Am. Chem. Soc., 119, 11108 (1997).
174
W. A. Moradi and S. L. Buchwald, J. Am. Chem. Soc., 123, 7996 (2001).