Page 756 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 756
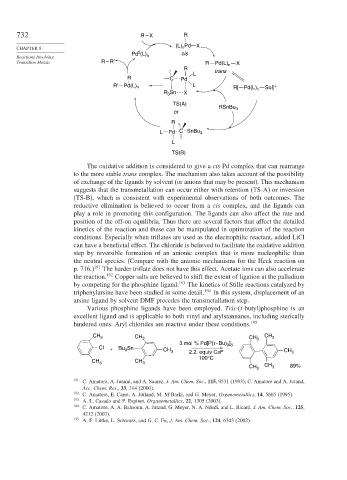
732 R – X R
(L) n Pd X
CHAPTER 8
0
Pd (L) cis
Reactions Involving n
Transition Metals R – R′ R Pd(L) n X
R
L trans
R C Pd
R′ Pd(L) n L R[ Pd(L) Sol] +
Sn n
R 3 X
TS(A)
RSnBu 3
or
R
L Pd CSnBu 3
L
TS(B)
The oxidative addition is considered to give a cis Pd complex that can rearrange
to the more stable trans complex. The mechanism also takes account of the possibility
of exchange of the ligands by solvent (or anions that may be present). This mechanism
suggests that the transmetallation can occur either with retention (TS-A) or inversion
(TS-B), which is consistent with experimental observations of both outcomes. The
reductive elimination is believed to occur from a cis complex, and the ligands can
play a role in promoting this configuration. The ligands can also affect the rate and
position of the off-on equilibria. Thus there are several factors that affect the detailed
kinetics of the reaction and these can be manipulated in optimization of the reaction
conditions. Especially when triflates are used as the electrophilic reactant, added LiCl
can have a beneficial effect. The chloride is believed to facilitate the oxidative addition
step by reversible formation of an anionic complex that is more nucleophilic than
the neutral species. (Compare with the anionic mechanisms for the Heck reaction on
p. 716.) 191 The harder triflate does not have this effect. Acetate ions can also accelerate
the reaction. 192 Copper salts are believed to shift the extent of ligation at the palladium
by competing for the phosphine ligand. 193 The kinetics of Stille reactions catalyzed by
triphenylarsine have been studied in some detail. 194 In this system, displacement of an
arsine ligand by solvent DMF precedes the transmetallation step.
Various phosphine ligands have been employed. Tris-(t-butyl)phosphine is an
excellent ligand and is applicable to both vinyl and arylstannanes, including sterically
hindered ones. Aryl chlorides are reactive under these conditions. 195
CH 3 CH 3 CH 3 CH 3
3 mol % Pd[P(t - Bu) ]
3 3
Cl + Bu Sn CH 3 2.2. equiv CsF CH 3
3
100°C
CH 3 CH 3
CH 3 CH 3 89%
191 C. Amatore, A. Jutand, and A. Suarez, J. Am. Chem. Soc., 115, 9531 (1993); C. Amatore and A. Jutand,
Acc. Chem. Res., 33, 314 (2000).
192
C. Amatore, E. Carre, A. Jutland, M. M’Barki, and G. Meyer, Organometallics, 14, 5605 (1995).
193 A. L. Casado and P. Espinet, Organometallics, 22, 1305 (2003).
194 C. Amatore, A. A. Bahsoun, A. Jutand, G. Meyer, N. A. Ndedi, and L. Ricard, J. Am. Chem. Soc., 125,
4212 (2003).
195
A. F. Littke, L. Schwarz, and G. C. Fu, J. Am. Chem. Soc., 124, 6343 (2002).