Page 760 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 760
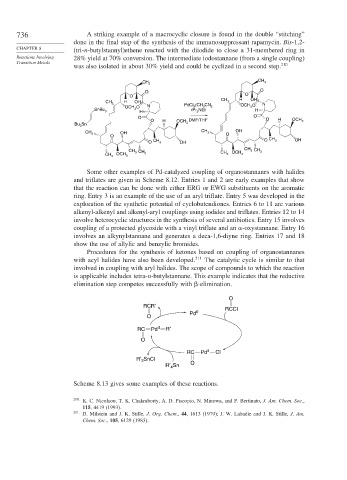
736 A striking example of a macrocyclic closure is found in the double “stitching”
done in the final step of the synthesis of the immunosuppressant rapamycin. Bis-1,2-
CHAPTER 8 (tri-n-butylstannyl)ethene reacted with the diiodide to close a 31-membered ring in
Reactions Involving 28% yield at 70% conversion. The intermediate iodostannane (from a single coupling)
Transition Metals 210
was also isolated in about 30% yield and could be cyclized in a second step.
CH 3
CH 3
O O
O O
H OH
H OH CH 3
CH 3 PdCl (CH CN OCH N
OCH O N 2 3 2 3 O
SnBu 3 i Pr NEt H
3 H 2
I O
O O O H OCH 3
3
Bu Sn I H OCH DMF/THF
3
CH OH CH 3 OH
3 O
O
O CH 3 OH O CH 3 OH
CH CH
3
CH 3 OCH 3 CH 3 CH 3 CH 3 OCH 3 3
Some other examples of Pd-catalyzed coupling of organostannanes with halides
and triflates are given in Scheme 8.12. Entries 1 and 2 are early examples that show
that the reaction can be done with either ERG or EWG substituents on the aromatic
ring. Entry 3 is an example of the use of an aryl triflate. Entry 5 was developed in the
exploration of the synthetic potential of cyclobutendiones. Entries 6 to 11 are various
alkenyl-alkenyl and alkenyl-aryl couplings using iodides and triflates. Entries 12 to 14
involve heterocyclic structures in the synthesis of several antibiotics. Entry 15 involves
coupling of a protected glycoside with a vinyl triflate and an -oxystannane. Entry 16
involves an alkynylstannane and generates a deca-1,6-diyne ring. Entries 17 and 18
show the use of allylic and benzylic bromides.
Procedures for the synthesis of ketones based on coupling of organostannanes
with acyl halides have also been developed. 211 The catalytic cycle is similar to that
involved in coupling with aryl halides. The scope of compounds to which the reaction
is applicable includes tetra-n-butylstannane. This example indicates that the reductive
elimination step competes successfully with -elimination.
O
RCR′ RCCl
Pd 0
O
RC Pd II R′
O
RC Pd II Cl
R′ SnCl O
3
R′ 4 Sn
Scheme 8.13 gives some examples of these reactions.
210 K. C. Nicolaou, T. K. Chakraborty, A. D. Piscopio, N. Minowa, and P. Bertinato, J. Am. Chem. Soc.,
115, 4419 (1993).
211
D. Milstein and J. K. Stille, J. Org. Chem., 44, 1613 (1979); J. W. Labadie and J. K. Stille, J. Am.
Chem. Soc., 105, 6129 (1983).