Page 765 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 765
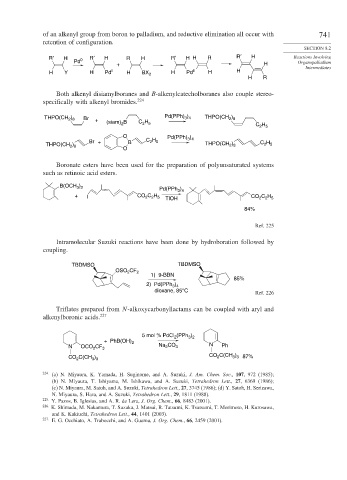
of an alkenyl group from boron to palladium, and reductive elimination all occur with 741
retention of configuration.
SECTION 8.2
R′ H 0 R′ H R H R′ H H R R′ H Reactions Involving
Pd Organopalladium
+ H
Intermediates
H Y H Pd II H BX 2 H Pd II H H
H R
Both alkenyl disiamylboranes and B-alkenylcatecholboranes also couple stereo-
specifically with alkenyl bromides. 224
THPO(CH ) Br + (siam) B C H Pd(PPh) ) THPO(CH ) C H
3 4
2 8
2 8
2
2 5
2 5
O Pd(PPh) )
Br + B C H 3 4 C H
2 5
) THPO(CH ) 2 5
THPO(CH 2 8 2 8
O
Boronate esters have been used for the preparation of polyunsaturated systems
such as retinoic acid esters.
B(OCH ) Pd(PPh )
3 2
3 4
+ I CO C H TlOH CO 2 2 5
C H
2 2 5
84%
Ref. 225
Intramolecular Suzuki reactions have been done by hydroboration followed by
coupling.
TBDMSO TBDMSO
CF
OSO 2 2
1) 9-BBN
85%
2) Pd(PPh )
3 4
dioxane, 85°C Ref. 226
Triflates prepared from N-alkoxycarbonyllactams can be coupled with aryl and
alkenylboronic acids. 227
5 mol % PdCl (PPh )
2
3 2
+ PhB(OH) 2
N OCO CF 3 Na CO 3 N Ph
2
2
CO C(CH )
C(CH ) 2 3 3 87%
CO 2 3 3
224
(a) N. Miyaura, K. Yamada, H. Suginome, and A. Suzuki, J. Am. Chem. Soc., 107, 972 (1985);
(b) N. Miyaura, T. Ishiyama, M. Ishikawa, and A. Suzuki, Tetrahedron Lett., 27, 6369 (1986);
(c) N. Miyaura, M. Satoh, and A. Suzuki, Tetrahedron Lett., 27, 3745 (1986); (d) Y. Satoh, H. Serizawa,
N. Miyaura, S. Hara, and A. Suzuki, Tetrahedron Lett., 29, 1811 (1988).
225 Y. Pazos, B. Iglesias, and A. R. de Lera, J. Org. Chem., 66, 8483 (2001).
226 K. Shimada, M. Nakamura, T. Suzuka, J. Matsui, R. Tatsumi, K. Tsutsumi, T. Morimoto, H. Kurosawa,
and K. Kakiuchi, Tetrahedron Lett., 44, 1401 (2003).
227
E. G. Occhiato, A. Trabocchi, and A. Guarna, J. Org. Chem., 66, 2459 (2001).