Page 759 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 759
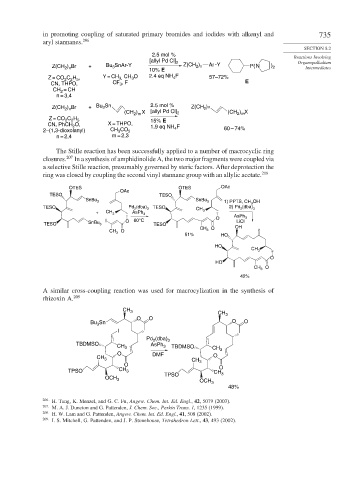
in promoting coupling of saturated primary bromides and iodides with alkenyl and 735
aryl stannanes. 206
SECTION 8.2
2.5 mol % Reactions Involving
[allyl Pd Cl] 2 Organopalladium
Z(CH ) Br + Bu SnAr-Y Z(CH ) Ar -Y P(N ) 2 Intermediates
2 n
3
2 n
10% E
Z = CO C H , Y = CH 3, CH 3 O 2.4 eq NH F 57–72%
4
2 2 5
CN, THPO, CF , F E
3
CH = CH
2
n = 3,4
Z(CH ) Br + Bu Sn 2.5 mol % Z(CH 2 )n
3
2 n
(CH ) X [allyl Pd Cl] 2 (CH ) X
2 m
2 m
Z = CO C H 15% E
2 2 5
CN, PhCH O, X = THPO, 1.9 eq NH F
2
2–(1,3-dioxolanyl) CH CO 2 4 60 – 74%
3
n = 2,4 m = 2,3
The Stille reaction has been successfully applied to a number of macrocyclic ring
closures. 207 In a synthesis of amphidinolide A, the two major fragments were coupled via
a selective Stille reaction, presumably governed by steric factors. After deprotection the
ring was closed by coupling the second vinyl stannane group with an allylic acetate. 208
OTES OTES OAc
OAc
TESO TESO
SnBu 3 SnBu 3 1) PPTS, CH OH
3
TESO Pd (dba) 3 TESO CH 2) Pd 2 (dba) 3
2
+ CH 3 AsPh 3 3
O AsPh 3
I O 60°C LiCl
TESO SnBu 3 TESO
O OH
O CH 3
CH 3
51% HO
HO
CH
3
O
HO
O
CH 3
42%
A similar cross-coupling reaction was used for macrocylization in the synthesis of
rhizoxin A. 209
CH 3 CH
O O 3
Bu 3 Sn O O
I
Pd (dba) 3
2
TBDMSO AsPh
CH 3 3 TBDMSO CH 3
O DMF
CH 3 CH O
O 3 O
TPSO CH 3
TPSO CH 3
OCH 3
OCH 3
48%
206
H. Tang, K. Menzel, and G. C. Fu, Angew. Chem. Int. Ed. Engl., 42, 5079 (2003).
207
M. A. J. Duncton and G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1235 (1999).
208 H. W. Lam and G. Pattenden, Angew. Chem. Int. Ed. Engl., 41, 508 (2002).
209
I. S. Mitchell, G. Pattenden, and J. P. Stonehouse, Tetrahedron Lett., 43, 493 (2002).