Page 822 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 822
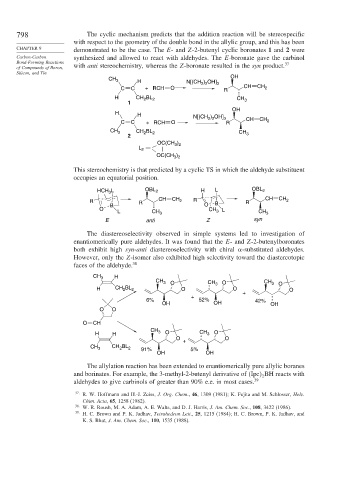
798 The cyclic mechanism predicts that the addition reaction will be stereospecific
with respect to the geometry of the double bond in the allylic group, and this has been
CHAPTER 9 demonstrated to be the case. The E- and Z-2-butenyl cyclic boronates 1 and 2 were
Carbon-Carbon synthesized and allowed to react with aldehydes. The E-boronate gave the carbinol
Bond-Forming Reactions 37
of Compounds of Boron, with anti stereochemistry, whereas the Z-boronate resulted in the syn product.
Silicon, and Tin
CH 3 H N[(CH ) OH] 3 OH
2 2
C C + RCH O R CH CH 2
H CH BL 2 CH 3
2
1
OH
H H N[(CH ) OH]
C C + RCH O 2 2 3 R CH CH 2
CH 3 CH BL 2
2
2 CH 3
OC(CH )
3 2
L 2
OC(CH )
3 2
This stereochemistry is that predicted by a cyclic TS in which the aldehyde substituent
occupies an equatorial position.
HCH L OBL 2 H L OBL 2
3
CH CH CH CH
R R 2 R R 2
B O B
O CH L
L CH 3 3 CH 3
E anti Z syn
The diastereoselectivity observed in simple systems led to investigation of
enantiomerically pure aldehydes. It was found that the E- and Z-2-butenylboronates
both exhibit high syn-anti diastereoselectivity with chiral -substituted aldehydes.
However, only the Z-isomer also exhibited high selectivity toward the diastereotopic
faces of the aldehyde. 38
CH 3 H
CH 3 O CH 3 O CH 3 O
H CH 2 BL 2 O O O
+
+
6% 52% 42%
OH OH OH
O O
O CH
CH 3 O
H H CH 3 O
O O
+
CH BL
CH 3 2 2 91% 5%
OH OH
The allylation reaction has been extended to enantiomerically pure allylic boranes
and borinates. For example, the 3-methyl-2-butenyl derivative of Ipc BH reacts with
2
aldehydes to give carbinols of greater than 90% e.e. in most cases. 39
37
R. W. Hoffmann and H.-J. Zeiss, J. Org. Chem., 46, 1309 (1981); K. Fujita and M. Schlosser, Helv.
Chim. Acta, 65, 1258 (1982).
38 W. R. Roush, M. A. Adam, A. E. Walts, and D. J. Harris, J. Am. Chem. Soc., 108, 3422 (1986).
39
H. C. Brown and P. K. Jadhav, Tetrahedron Lett., 25, 1215 (1984); H. C. Brown, P. K. Jadhav, and
K. S. Bhat, J. Am. Chem. Soc., 110, 1535 (1988).