Page 824 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 824
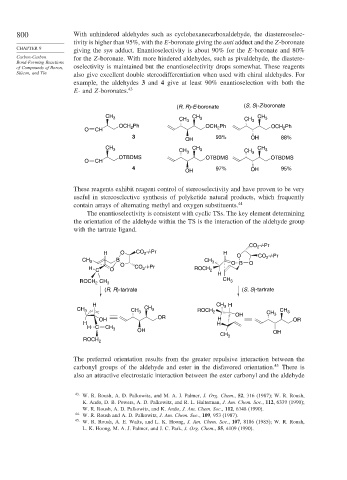
800 With unhindered aldehydes such as cyclohexanecarboxaldehyde, the diastereoselec-
tivity is higher than 95%, with the E-boronate giving the anti adduct and the Z-boronate
CHAPTER 9
giving the syn adduct. Enantioselectivity is about 90% for the E-boronate and 80%
Carbon-Carbon for the Z-boronate. With more hindered aldehydes, such as pivaldehyde, the diastere-
Bond-Forming Reactions
of Compounds of Boron, oselectivity is maintained but the enantioselectivity drops somewhat. These reagents
Silicon, and Tin also give excellent double stereodifferentiation when used with chiral aldehydes. For
example, the aldehydes 3 and 4 give at least 90% enantioselection with both the
E- and Z-boronates. 43
(R, R)-E-boronate (S, S)-Z-boronate
CH CH
CH 3 3 CH 3
CH 3 3
OCH Ph OCH Ph Ph
O CH 2 2 OCH 2
3 93% OH 88%
OH
CH 3 CH 3 CH 3 CH 3 CH 3
OTBDMS OTBDMS OTBDMS
O CH
4 97% OH 95%
OH
These reagents exhibit reagent control of stereoselectivity and have proven to be very
useful in stereoselective synthesis of polyketide natural products, which frequently
contain arrays of alternating methyl and oxygen substituents. 44
The enantioselectivity is consistent with cyclic TSs. The key element determining
the orientation of the aldehyde within the TS is the interaction of the aldehyde group
with the tartrate ligand.
CO -i-Pr
2
H O CO -i-Pr H O -i-Pr
2
CH 3 B CH 3 CO 2
O O B O
H C O CO -i-Pr ROCH 2
2
H
ROCH CH 3 CH 3
2
(R, R)-tartrate (S, S)-tartrate
H CH 3 H
CH 3 CH 3 CH 3 ROCH 2 CH 3 CH 3
OH OR H OH OR
H H
H C CH 3 OH
CH 3 OH
ROCH 2
The preferred orientation results from the greater repulsive interaction between the
carbonyl groups of the aldehyde and ester in the disfavored orientation. 45 There is
also an attractive electrostatic interaction between the ester carbonyl and the aldehyde
43 W. R. Roush, A. D. Palkowitz, and M. A. J. Palmer, J. Org. Chem., 52, 316 (1987); W. R. Roush,
K. Ando, D. B. Powers, A. D. Palkowitz, and R. L. Halterman, J. Am. Chem. Soc., 112, 6339 (1990);
W. R. Roush, A. D. Palkowitz, and K. Ando, J. Am. Chem. Soc., 112, 6348 (1990).
44 W. R. Roush and A. D. Palkowitz, J. Am. Chem. Soc., 109, 953 (1987).
45
W. R. Roush, A. E. Walts, and L. K. Hoong, J. Am. Chem. Soc., 107, 8186 (1985); W. R. Roush,
L. K. Hoong, M. A. J. Palmer, and J. C. Park, J. Org. Chem., 55, 4109 (1990).