Page 828 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 828
∗
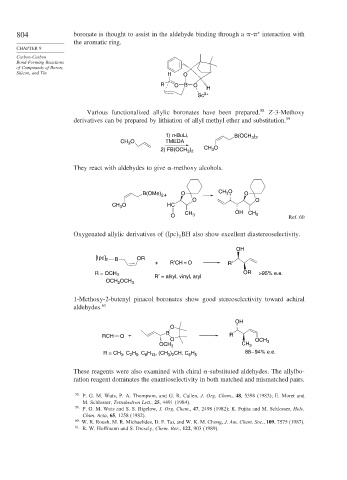
804 boronate is thought to assist in the aldehyde binding through a - interaction with
the aromatic ring.
CHAPTER 9
Carbon-Carbon
Bond-Forming Reactions
of Compounds of Boron,
Silicon, and Tin H O
R O B O
H
Sc 3+
Various functionalized allylic boronates have been prepared. 58 Z-3-Methoxy
derivatives can be prepared by lithiation of allyl methyl ether and substitution. 59
1) n-BuLi, B(OCH 3 2
)
CH O TMEDA
3
) CH 3 O
2) FB(OCH 3 2
They react with aldehydes to give -methoxy alcohols.
B(OMe) 2+ O CH 3 O O
O O
CH O HC
3
CH OH
O 3 CH 3 Ref. 60
Oxygenated allylic derivatives of Ipc BH also show excellent diastereoselectivity.
2
OH
[Ipc] 2 B OR
+ R′CH = O R′
OR
R = OCH 3 >95% e.e.
R′ = alkyl, vinyl, aryl
OCH OCH 3
2
1-Methoxy-2-butenyl pinacol boronates show good stereoselectivity toward achiral
aldehydes. 61
OH
O
B
RCH O + R
O OCH 3
OCH 3 CH 3
R = CH , C H , C H , (CH ) CH, C H 88 – 94% e.e.
6 5
2 5
6 13
3
3 2
These reagents were also examined with chiral -substituted aldehydes. The allylbo-
ration reagent dominates the enantioselectivity in both matched and mismatched pairs.
58
P. G. M. Wuts, P. A. Thompson, and G. R. Callen, J. Org. Chem., 48, 5398 (1983); E. Moret and
M. Schlosser, Tetrahedron Lett., 25, 4491 (1984).
59 P. G. M. Wuts and S. S. Bigelow, J. Org. Chem., 47, 2498 (1982); K. Fujita and M. Schlosser, Helv.
Chim. Acta, 65, 1258 (1982).
60 W. R. Roush, M. R. Michaelides, D. F. Tai, and W. K. M. Chong, J. Am. Chem. Soc., 109, 7575 (1987).
61
R. W. Hoffmann and S. Dresely, Chem. Ber., 122, 903 (1989).