Page 829 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 829
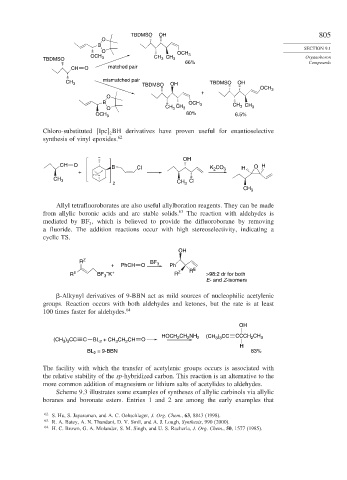
TBDMSO OH 805
O
B
SECTION 9.1
O OCH
OCH CH CH 3 Organoboron
TBDMSO 3 3 3
66% Compounds
CH O matched pair
mismatched pair
CH 3 OH TBDMSO OH
TBDMSO
OCH 3
+
O
B OCH 3 CH CH
3
O CH CH 3 3 3
60% 6.5%
OCH 3
Chloro-substituted Ipc BH derivatives have proven useful for enantioselective
2
synthesis of vinyl epoxides. 62
OH
CH O
B Cl K CO 3 H O H
2
+
CH 3 Cl
2 CH 3
CH 3
Allyl tetrafluoroborates are also useful allylboration reagents. They can be made
from allylic boronic acids and are stable solids. 63 The reaction with aldehydes is
mediated by BF , which is believed to provide the difluoroborane by removing
3
a fluoride. The addition reactions occur with high stereoselectivity, indicating a
cyclic TS.
OH
R Z BF
+ PhCH O 3 Ph
– +
R E BF K R Z R E >98:2 dr for both
3
E- and Z-isomers
-Alkynyl derivatives of 9-BBN act as mild sources of nucleophilic acetylenic
groups. Reaction occurs with both aldehydes and ketones, but the rate is at least
100 times faster for aldehydes. 64
OH
HOCH CH NH 2 (CH ) CC CCCH CH 3
2
2
2
(CH ) CC C BL + CH CH CH O 3 3
2
3 3
2
3
H
= 9-BBN 83%
BL 2
The facility with which the transfer of acetylenic groups occurs is associated with
the relative stability of the sp-hybridized carbon. This reaction is an alternative to the
more common addition of magnesium or lithium salts of acetylides to aldehydes.
Scheme 9.3 illustrates some examples of syntheses of allylic carbinols via allylic
boranes and boronate esters. Entries 1 and 2 are among the early examples that
62 S. Hu, S. Jayaraman, and A. C. Oehschlager, J. Org. Chem., 63, 8843 (1998).
63 R. A. Batey, A. N. Thandani, D. V. Smil, and A. J. Lough, Synthesis, 990 (2000).
64
H. C. Brown, G. A. Molander, S. M. Singh, and U. S. Racherla, J. Org. Chem., 50, 1577 (1985).