Page 827 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 827
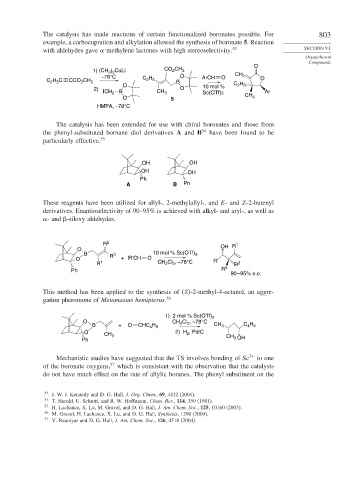
The catalysis has made reactions of certain functionalized boronates possible. For 803
example, a carbocupration and alkylation allowed the synthesis of boronate 5. Reaction
with aldehydes gave -methylene lactones with high stereoselectivity. 53 SECTION 9.1
Organoboron
Compounds
O
1) (CH ) CuLi CO CH 3
2
3 2
–78°C C H O ArCH O CH 2 O
C H C CCO CH 3 2 5 B
2
2 5
O 10 mol % C H
2 5
2) O
ICH 2 B CH 3 Sc(OTf)3 Ar
O 5 CH 3
HMPA, –78°C
The catalysis has been extended for use with chiral boronates and those from
the phenyl-substituted bornane diol derivatives A and B 54 have been found to be
particularly effective. 55
OH OH
OH OH
Ph
A B Ph
These reagents have been utilized for allyl-, 2-methylallyl-, and E- and Z-2-butenyl
derivatives. Enantioselectivity of 90–95% is achieved with alkyl- and aryl-, as well as
- and -siloxy aldehydes.
R 2 OH R 1
O
B 3 10 mol % Sc(OTf) 3
O R +R′CH O R′
R 1 CH Cl , –78°C R 2
2
2
Ph R 3
90 – 95% e.e.
This method has been applied to the synthesis of S -2-methyl-4-octanol, an aggre-
gation pheromone of Metamasius hemipterus. 56
1) 2 mol % Sc(OTf) 3
O CH Cl , –78°C
H
B + O CHC 4 9 2 2 CH 3 C H
4 9
O 2) H 2 , Pd/C
CH 3 CH
Ph 3 OH
Mechanistic studies have suggested that the TS involves bonding of Sc 3+ to one
of the boronate oxygens, 57 which is consistent with the observation that the catalysts
do not have much effect on the rate of allylic boranes. The phenyl substituent on the
53
J. W. J. Kennedy and D. G. Hall, J. Org. Chem., 69, 4412 (2004).
54
T. Herold, U. Schrott, and R. W. Hoffmann, Chem. Ber., 114, 359 (1981).
55 H. Lachance, X. Lu, M. Gravel, and D. G. Hall, J. Am. Chem. Soc., 125, 10160 (2003).
56 M. Gravel, H. Lachance, X. Lu, and D. G. Hall, Synthesis, 1290 (2004).
57
V. Rauniyar and D. G. Hall, J. Am. Chem. Soc., 126, 4518 (2004).