Page 832 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 832
808 Scheme 9.3. (Continued)
CHAPTER 9 a. R. W. Hoffmann and H.-J. Zeiss, J. Org. Chem., 46, 1309 (1981).
b. W. R. Roush and A. E. Walts, Tetrahedron Lett., 26, 3427 (1985); W. R. Roush, M. A. Adam, and D. J. Harris, J. Org.
Carbon-Carbon Chem., 50, 2000 (1985).
Bond-Forming Reactions c. Y. Yamamoto, K. Maruyama, T. Komatsu, and W. Ito, J. Org. Chem., 51, 886 (1986).
of Compounds of Boron, d. Y. Yamamoto, H. Yatagai, and K. Maruyama, J. Am. Chem. Soc., 103, 3229 (1981).
Silicon, and Tin e. W. R. Roush, M. R. Michaelides, D. F. Tai, and W. K. M. Chong, J. Am. Chem. Soc., 109, 7575 (1987).
f. C. Hertweck and W. Boland, Tetrahedron Lett., 53, 14651 (1997).
g. H. C. Brown, R. S. Randad, K. S. Bhat, M. Zaidlewicz, and U. S. Racherla, J. Am. Chem. Soc., 112, 2389 (1990).
h. R. W. Hoffmann, E. Haeberlin, and T. Rolide, Synthesis, 207 (2002).
i. L. K. Truesdale, D. Swanson, and R. C. Sun, Tetrahedron Lett., 26, 5009 (1985).
j. W. R. Roush, A. E. Walts, and L. K. Hoong, J. Am. Chem. Soc., 107, 8186 (1985).
k. Y. Yamamoto, S. Hara, and A. Suzuki, Synlett, 883 (1996).
l. W. R. Roush, J. A. Straub, and M. S. Van Nieuwenhze, J. Org. Chem., 56, 1636 (1985).
m. P. G. M. Wuts and S. S. Bigelow, J. Org. Chem., 53, 5023 (1988).
n. H. C. Brown, P. K. Jadhav, and K. S. Bhat, J. Am. Chem. Soc., 110, 1535 (1988).
o. M. Z. Hoemann, K. A. Agrios, and J. Aube, Tetrahedron, 53, 11087 (1997).
p. K. C. Nicolaou, M. E. Bunnage, and K. Koide, Chem. Eur. J., 1, 454 (1995).
q. A. L. Smith, E. N. Pitsinos, C.-K. Hwang, Y. Mizuno, H. Saimoto, G. R. Scarlato, T. Suzuki, and K. C. Nicolaou, J.
Am. Chem. Soc., 115, 7612 (1993).
r. T. Sunazuka, T. Nagamitsu, K. Matsuzaki, H. Tanaka, S. Omura, and A. B. Smith, III, J. Am. Chem. Soc., 115,
5302 (1993).
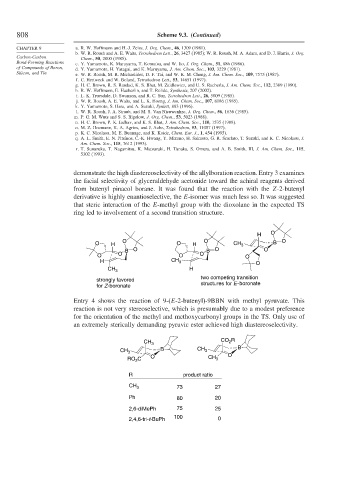
demonstrate the high diastereoselectivity of the allylboration reaction. Entry 3 examines
the facial selectivity of glyceraldehyde acetonide toward the achiral reagents derived
from butenyl pinacol borane. It was found that the reaction with the Z-2-butenyl
derivative is highly enantioselective, the E-isomer was much less so. It was suggested
that steric interaction of the E-methyl group with the dioxolane in the expected TS
ring led to involvement of a second transition structure.
H O
O O O
O H O H CH 3 B
B O B O O
O O O O O
H CH 3
O
CH 3 H
two competing transition
strongly favored
for Z- boronate structures for E - boronate
Entry 4 shows the reaction of 9-(E-2-butenyl)-9BBN with methyl pyruvate. This
reaction is not very stereoselective, which is presumably due to a modest preference
for the orientation of the methyl and methoxycarbonyl groups in the TS. Only use of
an extremely sterically demanding pyruvic ester achieved high diastereoselectivity.
CH 3 CO R
2
CH 3 B CH 3 B
RO C O CH 3 O
2
R product ratio
CH 3 73 27
Ph 80 20
2,6-diMePh 75 25
100
2,4,6-tri-t-BuPh 0