Page 823 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 823
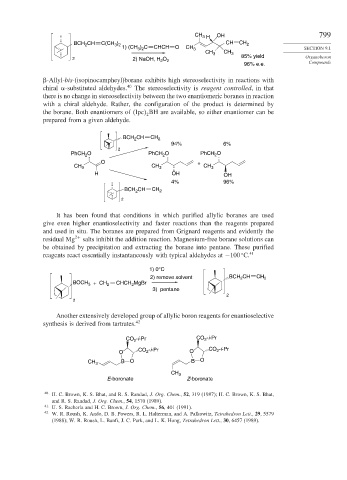
CH 3 H OH 799
BCH CH C(CH ) CH CH 2
3 2
2
1) (CH ) C CHCH O CH 3 SECTION 9.1
3 2
CH 3 CH 3
2 2) NaOH, H 2 O 85% yield Organoboron
2
96% e.e. Compounds
-Allyl-bis-(isopinocampheyl)borane exhibits high stereoselectivity in reactions with
chiral -substituted aldehydes. 40 The stereoselectivity is reagent controlled, in that
there is no change in stereoselectivity between the two enantiomeric boranes in reaction
with a chiral aldehyde. Rather, the configuration of the product is determined by
the borane. Both enantiomers of Ipc BH are available, so either enantiomer can be
2
prepared from a given aldehyde.
BCH CH CH 2
2
94% 6%
2
PhCH O PhCH O PhCH O
2
2
2
O +
CH 3 CH 3 CH 3
H OH OH
4% 96%
BCH CH CH 2
2
2
It has been found that conditions in which purified allylic boranes are used
give even higher enantioselectivity and faster reactions than the reagents prepared
and used in situ. The boranes are prepared from Grignard reagents and evidently the
residual Mg 2+ salts inhibit the addition reaction. Magnesium-free borane solutions can
be obtained by precipitation and extracting the borane into pentane. These purified
reagents react essentially instantaneously with typical aldehydes at −100 C. 41
1) 0°C
2) remove solvent BCH 2 CH CH 2
BOCH + CH 2 CHCH MgBr
3
2
3) pentane
2
2
Another extensively developed group of allylic boron reagents for enantioselective
synthesis is derived from tartrates. 42
2
CO 2 -i-Pr CO -i-Pr
CO -i-Pr CO -i-Pr
O 2 O 2
CH 3 B O B O
CH 3
E-boronate Z-boronate
40
H. C. Brown, K. S. Bhat, and R. S. Randad, J. Org. Chem., 52, 319 (1987); H. C. Brown, K. S. Bhat,
and R. S. Randad, J. Org. Chem., 54, 1570 (1989).
41 U. S. Racherla and H. C. Brown, J. Org. Chem., 56, 401 (1991).
42
W. R. Roush, K. Ando, D. B. Powers, R. L. Halterman, and A. Palkowitz, Tetrahedron Lett., 29, 5579
(1988); W. R. Roush, L. Banfi, J. C. Park, and L. K. Hong, Tetrahedron Lett., 30, 6457 (1989).