Page 825 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 825
46
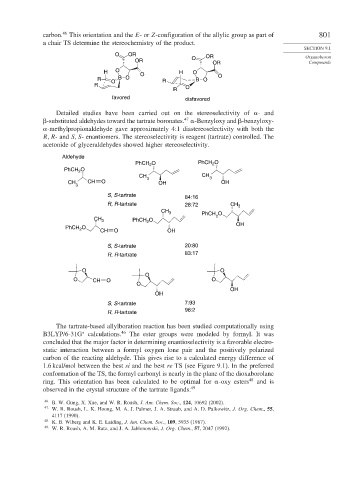
carbon. This orientation and the E-or Z-configuration of the allylic group as part of 801
a chair TS determine the stereochemistry of the product.
SECTION 9.1
O OR OR Organoboron
OR O
OR Compounds
H O O H O
R O B O R B O O
R
R O
favored disfavored
Detailed studies have been carried out on the stereoselectivity of - and
47
-substituted aldehydes toward the tartrate boronates. -Benzyloxy and -benzyloxy-
-methylpropionaldehyde gave approximately 4:1 diastereoselectivity with both the
R
R- and S
S- enantiomers. The stereoselectivity is reagent (tartrate) controlled. The
acetonide of glyceraldehydes showed higher stereoselectivity.
Aldehyde
PhCH O PhCH O
2
2
PhCH O
2
CH 3 CH 3
CH CH O OH OH
3
S, S-tartrate 84:16
R, R-tartrate 28:72 CH 3
CH 3 PhCH O
CH 3 PhCH 2 O 2
OH
PhCH O CH O OH
2
S, S-tartrate 20:80
R, R-tartrate 83:17
O O
O
O CH O O
O
OH
OH
S, S-tartrate 7:93
98:2
R, R-tartrate
The tartrate-based allylboration reaction has been studied computationally using
B3LYP/6-31G calculations. 46 The ester groups were modeled by formyl. It was
∗
concluded that the major factor in determining enantioselectivity is a favorable electro-
static interaction between a formyl oxygen lone pair and the positively polarized
carbon of the reacting aldehyde. This gives rise to a calculated energy difference of
1.6 kcal/mol between the best si and the best re TS (see Figure 9.1). In the preferred
conformation of the TS, the formyl carbonyl is nearly in the plane of the dioxaborolane
ring. This orientation has been calculated to be optimal for -oxy esters 48 and is
observed in the crystal structure of the tartrate ligands. 49
46
B. W. Gung, X. Xue, and W. R. Roush, J. Am. Chem. Soc., 124, 10692 (2002).
47
W. R. Roush, L. K. Hoong, M. A. J. Palmer, J. A. Straub, and A. D. Palkowitz, J. Org. Chem., 55,
4117 (1990).
48 K. B. Wiberg and K. E. Laiding, J. Am. Chem. Soc., 109, 5935 (1987).
49
W. R. Roush, A. M. Ratz, and J. A. Jablonowski, J. Org. Chem., 57, 2047 (1992).