Page 183 - Advanced Thermodynamics for Engineers, Second Edition
P. 183
170 CHAPTER 8 EQUATIONS OF STATE
25
eqn 8.25
eqn 8.27
20
Pressure, p / (bar) 15
10
5
0
0.001 0.01 0.1 1.0
3
Specific volume, v/ (m /kg)
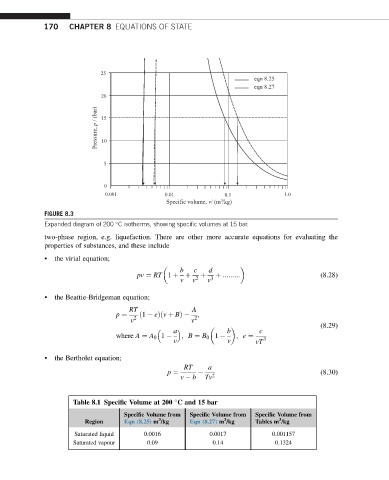
FIGURE 8.3
Expanded diagram of 200 C isotherms, showing specific volumes at 15 bar.
two-phase region, e.g. liquefaction. There are other more accurate equations for evaluating the
properties of substances, and these include
• the virial equation;
b c d
pv ¼ RT 1 þ þ 2 þ 3 þ ::::::::: (8.28)
v v v
• the Beattie-Bridgeman equation;
RT A
p ¼ 2 ð1 eÞðv þ BÞ 2 ;
v v
(8.29)
a b c
where A ¼ A 0 1 ; B ¼ B 0 1 ; e ¼ 3
v v vT
• the Bertholet equation;
RT a
p ¼ (8.30)
v b Tv 2
Table 8.1 Specific Volume at 200 C and 15 bar
Specific Volume from Specific Volume from Specific Volume from
3
3
3
Region Eqn (8.25) m /kg Eqn (8.27) m /kg Tables m /kg
Saturated liquid 0.0016 0.0017 0.001157
Saturated vapour 0.09 0.14 0.1324