Page 284 - Advanced Thermodynamics for Engineers, Second Edition
P. 284
12.10 DISSOCIATION CALCULATIONS FOR THE EVALUATION 273
0.7
0.6
Degree of dissociation, α 0.4 Carbon dioxide
0.5
0.3
Hydrogen
0.2
0.1
0
1000 1200 1400 1600 1800 2000
Temperature, T / (K)
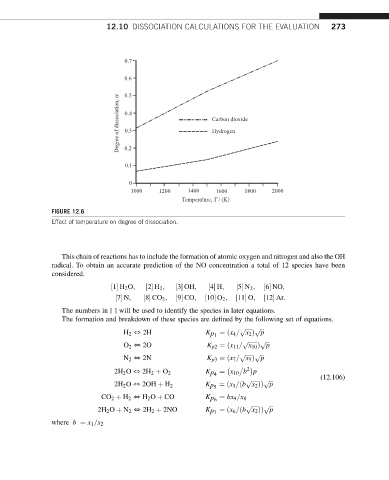
FIGURE 12.6
Effect of temperature on degree of dissociation.
This chain of reactions has to include the formation of atomic oxygen and nitrogen and also the OH
radical. To obtain an accurate prediction of the NO concentration a total of 12 species have been
considered.
½1 H 2 O; ½2 H 2 ; ½3 OH; ½4 H; ½5 N 2 ; ½6 NO;
½7 N; ½8 CO 2 ; ½9 CO; ½10 O 2 ; ½11 O; ½12 Ar:
The numbers in [ ] will be used to identify the species in later equations.
The formation and breakdown of these species are defined by the following set of equations.
ffiffiffi
ffiffiffiffiffi p
p
H 2 5 2H Kp 1 ¼ðx 4 = x 2 Þ p
ffiffiffi
ffiffiffiffiffiffi p
O 2 5 2O K p2 ¼ðx 11 = x 10 Þ p
p
ffiffiffi
p
ffiffiffiffiffi p
N 2 5 2N K p3 ¼ðx 7 = x 5 Þ p
2
2H 2 O 5 2H 2 þ O 2 K p 4 ¼ x 10 b p
(12.106)
ffiffiffi
ffiffiffiffiffi p
¼ðx 3 =ðb x 2 ÞÞ p
p
2H 2 O 5 2OH þ H 2 K p 5
CO 2 þ H 2 5 H 2 O þ CO K p 6 ¼ bx 9 =x 8
ffiffiffi
ffiffiffiffiffi p
p
2H 2 O þ N 2 5 2H 2 þ 2NO K p 7 ¼ðx 6 =ðb x 2 ÞÞ p
where b ¼ x 1 =x 2