Page 119 - Advanced thermodynamics for engineers
P. 119
5.3 RANKINE CYCLE 105
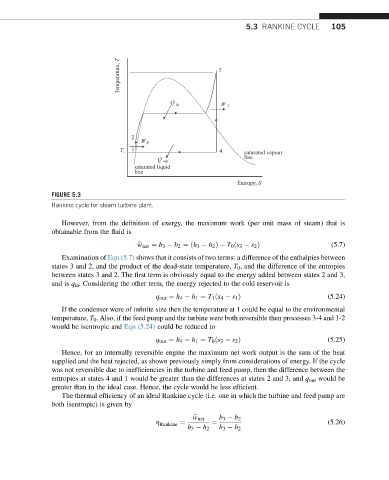
Temperature, T 3
Q in W T
2
W P
T 1 4 saturated vapour
line
Q out
saturated liquid
line
Entropy, S
FIGURE 5.3
Rankine cycle for steam turbine plant.
However, from the definition of exergy, the maximum work (per unit mass of steam) that is
obtainable from the fluid is
b w net ¼ b 3 b 2 ¼ðh 3 h 2 Þ T 0 ðs 3 s 2 Þ (5.7)
Examination of Eqn (5.7) shows that it consists of two terms: a difference of the enthalpies between
states 3 and 2, and the product of the dead-state temperature, T 0 , and the difference of the entropies
between states 3 and 2. The first term is obviously equal to the energy added between states 2 and 3,
and is q in . Considering the other term, the energy rejected to the cold reservoir is
q out ¼ h 4 h 1 ¼ T 1 ðs 4 s 1 Þ (5.24)
If the condenser were of infinite size then the temperature at 1 could be equal to the environmental
temperature, T 0 . Also, if the feed pump and the turbine were both reversible then processes 3-4 and 1-2
would be isentropic and Eqn (5.24) could be reduced to
q out ¼ h 4 h 1 ¼ T 0 ðs 3 s 2 Þ (5.25)
Hence, for an internally reversible engine the maximum net work output is the sum of the heat
supplied and the heat rejected, as shown previously simply from considerations of energy. If the cycle
was not reversible due to inefficiencies in the turbine and feed pump, then the difference between the
entropies at states 4 and 1 would be greater than the differences at states 2 and 3, and q out would be
greater than in the ideal case. Hence, the cycle would be less efficient.
The thermal efficiency of an ideal Rankine cycle (i.e. one in which the turbine and feed pump are
both isentropic) is given by
b w net b 3 b 2
h Rankine ¼ ¼ (5.26)
h 3 h 2 h 3 h 2