Page 121 - Advanced thermodynamics for engineers
P. 121
5.4 EXAMPLES 107
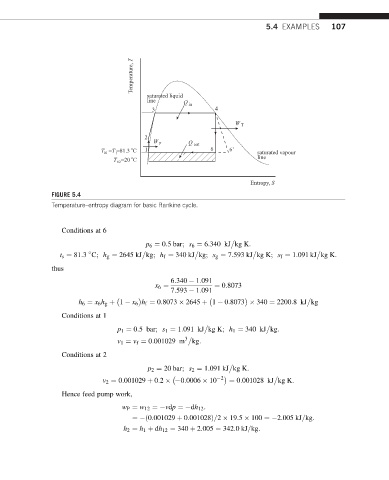
Temperature, T
saturated liquid
line Q in
3 4
W T
2
W P Q out
o
T =T =81.3 C 1 6 6 ' saturated vapour
01
1
o
T =20 C line
02
Entropy, S
FIGURE 5.4
Temperature–entropy diagram for basic Rankine cycle.
Conditions at 6
p 6 ¼ 0:5 bar; s 6 ¼ 6:340 kJ kg K:
t s ¼ 81:3 C; h g ¼ 2645 kJ kg; h f ¼ 340 kJ kg; s g ¼ 7:593 kJ kg K; s f ¼ 1:091 kJ kg K:
thus
6:340 1:091
x 6 ¼ ¼ 0:8073
7:593 1:091
h 6 ¼ x 6 h g þ 1 x 6 h f ¼ 0:8073 2645 þ 1 0:8073 340 ¼ 2200:8kJ kg
Conditions at 1
p 1 ¼ 0:5 bar; s 1 ¼ 1:091 kJ kg K; h 1 ¼ 340 kJ kg:
v 1 ¼ v f ¼ 0:001029 m 3 kg:
Conditions at 2
p 2 ¼ 20 bar; s 2 ¼ 1:091 kJ kg K:
v 2 ¼ 0:001029 þ 0:2 0:0006 10 2 ¼ 0:001028 kJ kg K:
Hence feed pump work,
w P ¼ w 12 ¼ vdp ¼ dh 12 :
¼ ð0:001029 þ 0:001028Þ=2 19:5 100 ¼ 2:005 kJ=kg:
h 2 ¼ h 1 þ dh 12 ¼ 340 þ 2:005 ¼ 342:0kJ=kg: