Page 56 - Advanced thermodynamics for engineers
P. 56
40 CHAPTER 3 ENGINE CYCLES AND THEIR EFFICIENCIES
(a) (b) T 5
Temperature, T Temperature, T
T 3 Q in 4 T 3 Q in 4
W T
W T
2 W P 2 W P
1 1 6' 6
T 1 6 T 1
Q out Q out
Saturated Saturated Saturated Saturated
liquid line vapour line liquid line vapour line
Specific entropy, s Specific entropy, s
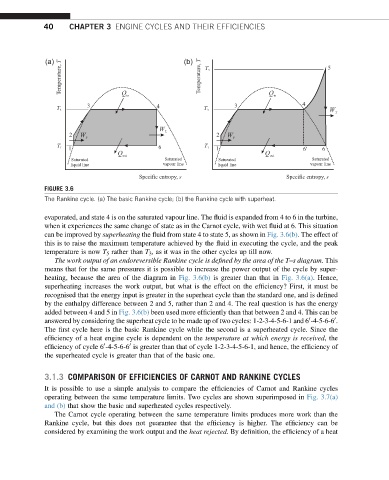
FIGURE 3.6
The Rankine cycle. (a) The basic Rankine cycle; (b) the Rankine cycle with superheat.
evaporated, and state 4 is on the saturated vapour line. The fluid is expanded from 4 to 6 in the turbine,
when it experiences the same change of state as in the Carnot cycle, with wet fluid at 6. This situation
can be improved by superheating the fluid from state 4 to state 5, as shown in Fig. 3.6(b). The effect of
this is to raise the maximum temperature achieved by the fluid in executing the cycle, and the peak
temperature is now T 5 rather than T 3 , as it was in the other cycles up till now.
The work output of an endoreversible Rankine cycle is defined by the area of the T–s diagram. This
means that for the same pressures it is possible to increase the power output of the cycle by super-
heating, because the area of the diagram in Fig. 3.6(b) is greater than that in Fig. 3.6(a). Hence,
superheating increases the work output, but what is the effect on the efficiency? First, it must be
recognised that the energy input is greater in the superheat cycle than the standard one, and is defined
by the enthalpy difference between 2 and 5, rather than 2 and 4. The real question is has the energy
added between 4 and 5 in Fig. 3.6(b) been used more efficiently than that between 2 and 4. This can be
answered by considering the superheat cycle to be made up of two cycles: 1-2-3-4-5-6-1 and 6 -4-5-6-6 .
0
0
The first cycle here is the basic Rankine cycle while the second is a superheated cycle. Since the
efficiency of a heat engine cycle is dependent on the temperature at which energy is received, the
efficiency of cycle 6 -4-5-6-6 is greater than that of cycle 1-2-3-4-5-6-1, and hence, the efficiency of
0
0
the superheated cycle is greater than that of the basic one.
3.1.3 COMPARISON OF EFFICIENCIES OF CARNOT AND RANKINE CYCLES
It is possible to use a simple analysis to compare the efficiencies of Carnot and Rankine cycles
operating between the same temperature limits. Two cycles are shown superimposed in Fig. 3.7(a)
and (b) that show the basic and superheated cycles respectively.
The Carnot cycle operating between the same temperature limits produces more work than the
Rankine cycle, but this does not guarantee that the efficiency is higher. The efficiency can be
considered by examining the work output and the heat rejected. By definition, the efficiency of a heat