Page 291 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 291
1522_book.fm Page 262 Thursday, November 13, 2003 9:58 AM
35
[P - Cl - H] −
315
100 PETN
90
80
70
Relative abundance 60 P = [M + Cl] −
50
35
351
40
2
[ONO + NO 2 + H] −
30
−
20 [P - 35 Cl - 2ONO ]
2
192
10
109
0
100 120 140 160 180 200 220 240 260 280 300 320 340
m/z
-
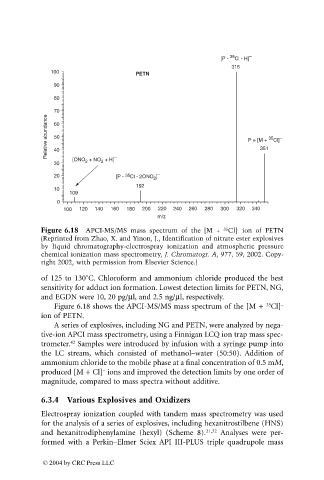
Figure 6.18 APCI-MS/MS mass spectrum of the [M + Cl] ion of PETN
35
(Reprinted from Zhao, X. and Yinon, J., Identification of nitrate ester explosives
by liquid chromatography-electrospray ionization and atmospheric pressure
chemical ionization mass spectrometry, J. Chromatogr. A, 977, 59, 2002. Copy-
right 2002, with permission from Elsevier Science.)
of 125 to 130˚C. Chloroform and ammonium chloride produced the best
sensitivity for adduct ion formation. Lowest detection limits for PETN, NG,
and EGDN were 10, 20 pg/ml, and 2.5 ng/ml, respectively.
35
Figure 6.18 shows the APCI-MS/MS mass spectrum of the [M + Cl] –
ion of PETN.
A series of explosives, including NG and PETN, were analyzed by nega-
tive-ion APCI mass spectrometry, using a Finnigan LCQ ion trap mass spec-
42
trometer. Samples were introduced by infusion with a syringe pump into
the LC stream, which consisted of methanol–water (50:50). Addition of
ammonium chloride to the mobile phase at a final concentration of 0.5 mM,
–
produced [M + Cl] ions and improved the detection limits by one order of
magnitude, compared to mass spectra without additive.
6.3.4 Various Explosives and Oxidizers
Electrospray ionization coupled with tandem mass spectrometry was used
for the analysis of a series of explosives, including hexanitrostilbene (HNS)
and hexanitrodiphenylamine (hexyl) (Scheme 8). 21,32 Analyses were per-
formed with a Perkin–Elmer Sciex API III-PLUS triple quadrupole mass
© 2004 by CRC Press LLC