Page 292 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 292
1522_book.fm Page 263 Thursday, November 13, 2003 9:58 AM
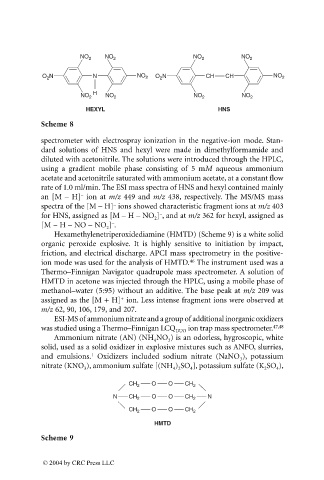
NO 2 NO 2 NO 2 NO 2
O N N NO 2 O N CH CH NO 2
2
2
NO 2 H NO 2 NO 2 NO 2
HEXYL HNS
Scheme 8
spectrometer with electrospray ionization in the negative-ion mode. Stan-
dard solutions of HNS and hexyl were made in dimethylformamide and
diluted with acetonitrile. The solutions were introduced through the HPLC,
using a gradient mobile phase consisting of 5 mM aqueous ammonium
acetate and acetonitrile saturated with ammonium acetate, at a constant flow
rate of 1.0 ml/min. The ESI mass spectra of HNS and hexyl contained mainly
an [M – H] ion at m/z 449 and m/z 438, respectively. The MS/MS mass
–
–
spectra of the [M – H] ions showed characteristic fragment ions at m/z 403
for HNS, assigned as [M – H – NO ] , and at m/z 362 for hexyl, assigned as
–
2
[M – H – NO – NO ] .
–
2
Hexamethylenetriperoxidediamine (HMTD) (Scheme 9) is a white solid
organic peroxide explosive. It is highly sensitive to initiation by impact,
friction, and electrical discharge. APCI mass spectrometry in the positive-
46
ion mode was used for the analysis of HMTD. The instrument used was a
Thermo–Finnigan Navigator quadrupole mass spectrometer. A solution of
HMTD in acetone was injected through the HPLC, using a mobile phase of
methanol–water (5:95) without an additive. The base peak at m/z 209 was
assigned as the [M + H] ion. Less intense fragment ions were observed at
+
m/z 62, 90, 106, 179, and 207.
ESI-MS of ammonium nitrate and a group of additional inorganic oxidizers
was studied using a Thermo–Finnigan LCQ DUO ion trap mass spectrometer. 47,48
Ammonium nitrate (AN) (NH NO ) is an odorless, hygroscopic, white
4
3
solid, used as a solid oxidizer in explosive mixtures such as ANFO, slurries,
and emulsions. Oxidizers included sodium nitrate (NaNO ), potassium
1
3
nitrate (KNO ), ammonium sulfate [(NH ) SO ], potassium sulfate (K SO ),
4 2
4
3
2
4
CH 2 O O CH 2
N CH 2 O O CH 2 N
CH 2 O O CH 2
HMTD
Scheme 9
© 2004 by CRC Press LLC