Page 296 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 296
1522_book.fm Page 267 Thursday, November 13, 2003 9:58 AM
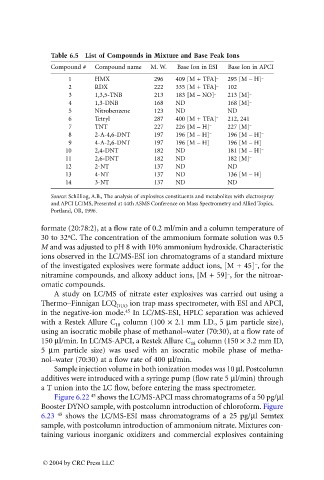
Table 6.5 List of Compounds in Mixture and Base Peak Ions
Compound # Compound name M. W. Base Ion in ESI Base Ion in APCI
1 HMX 296 409 [M + TFA] – 295 [M – H] –
2 RDX 222 335 [M + TFA] – 102
3 1,3,5-TNB 213 183 [M – NO] – 213 [M] –
4 1,3-DNB 168 ND 168 [M] –
5 Nitrobenzene 123 ND ND
6 Tetryl 287 400 [M + TFA] – 212, 241
7 TNT 227 226 [M – H] – 227 [M] –
8 2-A-4,6-DNT 197 196 [M – H] – 196 [M – H] –
9 4-A-2,6-DNT 197 196 [M – H] – 196 [M – H] –
10 2,4-DNT 182 ND 181 [M – H] –
11 2,6-DNT 182 ND 182 [M] –
12 2-NT 137 ND ND
13 4-NT 137 ND 136 [M – H] –
14 3-NT 137 ND ND
Source: Schilling, A.B., The analysis of explosives constituents and metabolites with electrospray
and APCI LC/MS, Presented at 44th ASMS Conference on Mass Spectrometry and Allied Topics,
Portland, OR, 1996.
formate (20:78:2), at a flow rate of 0.2 ml/min and a column temperature of
o
30 to 32 C. The concentration of the ammonium formate solution was 0.5
M and was adjusted to pH 8 with 10% ammonium hydroxide. Characteristic
ions observed in the LC/MS-ESI ion chromatograms of a standard mixture
–
of the investigated explosives were formate adduct ions, [M + 45] , for the
–
nitramine compounds, and alkoxy adduct ions, [M + 59] , for the nitroar-
omatic compounds.
A study on LC/MS of nitrate ester explosives was carried out using a
Thermo–Finnigan LCQ DUO ion trap mass spectrometer, with ESI and APCI,
45
in the negative-ion mode. In LC/MS-ESI, HPLC separation was achieved
with a Restek Allure C column (100 ¥ 2.1 mm I.D., 5 mm particle size),
18
using an isocratic mobile phase of methanol–water (70:30), at a flow rate of
150 ml/min. In LC/MS-APCI, a Restek Allure C column (150 ¥ 3.2 mm ID,
18
5 mm particle size) was used with an isocratic mobile phase of metha-
nol–water (70:30) at a flow rate of 400 ml/min.
Sample injection volume in both ionization modes was 10 ml. Postcolumn
additives were introduced with a syringe pump (flow rate 5 ml/min) through
a T union into the LC flow, before entering the mass spectrometer.
45
Figure 6.22 shows the LC/MS-APCI mass chromatograms of a 50 pg/ml
Booster DYNO sample, with postcolumn introduction of chloroform. Figure
6.23 45 shows the LC/MS-ESI mass chromatograms of a 25 pg/ml Semtex
sample, with postcolumn introduction of ammonium nitrate. Mixtures con-
taining various inorganic oxidizers and commercial explosives containing
© 2004 by CRC Press LLC