Page 295 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 295
1522_book.fm Page 266 Thursday, November 13, 2003 9:58 AM
+
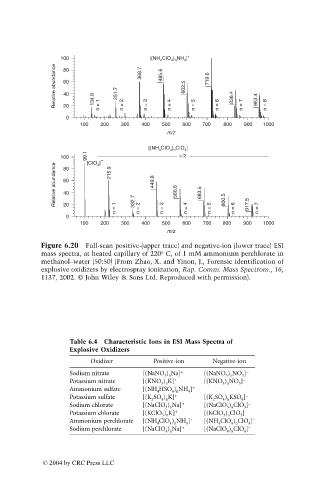
100 [(NH CIO ) NH ]
4
4 n
4
Relative abundance 60 134.8 251.7 368.7 485.6 602.5 719.6 836.4 953.4
80
40
20
0 n = 1 n = 2 n = 3 n = 4 n = 5 n = 6 n = 7 n = 8
100 200 300 400 500 600 700 800 900 1000
m/z
−
[(NH CIO ) CIO ]
4
4 n
4
99.1 × 2
100 [CIO ] −
Relative abundance 60 215.9 449.8 566.6 683.5
4
80
40
n = 1 332.7 n = 2 n = 3 n = 4 n = 5 800.5 n = 6 917.5 n = 7
20
0
100 200 300 400 500 600 700 800 900 1000
m/z
Figure 6.20 Full-scan positive-(upper trace) and negative-ion (lower trace) ESI
o
mass spectra, at heated capillary of 220 C, of 1 mM ammonium perchlorate in
methanol–water (50:50) (From Zhao, X. and Yinon, J., Forensic identification of
explosive oxidizers by electrospray ionization, Rap. Comm. Mass Spectrom., 16,
1137, 2002. © John Wiley & Sons Ltd. Reproduced with permission).
Table 6.4 Characteristic Ions in ESI Mass Spectra of
Explosive Oxidizers
Oxidizer Positive-ion Negative-ion
Sodium nitrate [(NaNO 3 ) n Na] + [(NaNO 3 ) n NO 3 ] –
Potassium nitrate [(KNO 3 ) n K] + [(KNO 3 ) n NO 3 ] –
Ammonium sulfate [(NH 4 HSO 4 ) n NH 4 ] +
Potassium sulfate [(K 2 SO 4 ) n K] + [(K 2 SO 4 ) n KSO 4 ] –
Sodium chlorate [(NaClO 3 ) n Na] + [(NaClO 3 ) n ClO 3 ] –
Potassium chlorate [(KClO 3 ) n K] + [(KClO 3 ) n ClO 3 ] –
Ammonium perchlorate [(NH 4 ClO 4 ) n NH 4 ] + [(NH 4 ClO 4 ) n ClO 4 ] –
Sodium perchlorate [(NaClO 4 ) n Na] + [(NaClO 4 ) n ClO 4 ] –
© 2004 by CRC Press LLC