Page 238 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 238
214 7 Combustion Process and Air Emission Formation
O 2 þ N $ NO þ O ð7:70Þ
HCN þ OH $ CN þ H 2 O ð7:71Þ
CN þ O 2 $ NO þ CO ð7:72Þ
Prompt NO is formed only in a combustion zone of the flame where the com-
bustion is incomplete and the hydrocarbon radicals are present [3]. These reactions
take place very fast, thus it is known as prompt NO. The formation of prompt NO does
not depend on temperature as significantly as the thermal NO. The prompt NO is
formed mainly under lower temperature conditions during a short residence time [24].
7.7.1.3 NO Through Intermediate Component N 2 O
The third mechanism is through N 2 O[20, 32], also known as laughing gas, pro-
duced by the reaction between oxygen atoms and N 2 :
O þ N 2 þ M $ N 2 O þ M ð7:73Þ
N 2 O þ O $ 2NO ð7:74Þ
where M can be any coexisting gas compound. Depending on the condition, N 2 O
could react again either forward to NO or backward to N 2 , and the later often
dominates. In general, the formation of NO increases with the air to fuel ratio and
temperature.
N 2 O is one of the greenhouse gases (GHGs). It can also be formed through other
mechanisms, including oxidation of HCN and oxidation of char residue. Part of the
volatile cyanide compounds of the fuel nitrogen, such as HCN, is oxidized
homogeneously to N 2 O through the following reactions:
HCN þ O $ NCO þ H ð7:75Þ
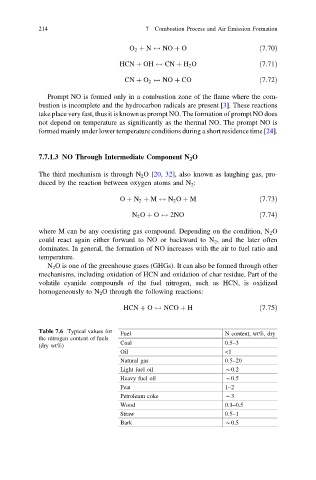
Table 7.6 Typical values for Fuel N content, wt%, dry
the nitrogen content of fuels
Coal 0.5–3
(dry wt%)
Oil <1
Natural gas 0.5–20
Light fuel oil *0.2
Heavy fuel oil *0.5
Peat 1–2
Petroleum coke *3
Wood 0.1–0.5
Straw 0.5–1
Bark *0.5