Page 239 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 239
7.7 NO x 215
NCO þ NO $ N 2 O þ CO ð7:76Þ
The reaction is also very sensitive to temperature and it slows down significantly
when the temperature rises. The reaction stops when the temperature is above
950 °C, and the NCO radicals are converted to NO.
7.7.1.4 Fuel NO
Most fuels contain nitrogen element, the NO originated from this part of nitrogen is
referred to as fuel NO [19, 33, 37, 44]. The amount of nitrogen in fuel varies with
the fuel type. As summarized in Table 7.6 [51], typical coal and oil contain
chemically bound organic nitrogen, which is different from that found in natural
gas. Depending on the refinery process, some natural gases contain virtually no
nitrogen but others have quite a lot of nitrogen in form of N 2 . Unlike fossil fuels,
biomass is characterized with high nitrogen.
Although the amount of nitrogen in fuel is relatively small, the fuel nitrogen is
much more reactive compared to the nitrogen present in the combustion air.
Consequently, the formation of fuel NO from a nitrogen-rich fuel is higher than that
from a nitrogen-lean fuel. Sometimes, as much as 80 % of the NO in the flue gas of
a coal-firing furnace is produced from fuel nitrogen [33]. The fuel NO is sensitive to
stoichiometry rather than the temperature because it forms readily at quite low
temperatures [7].
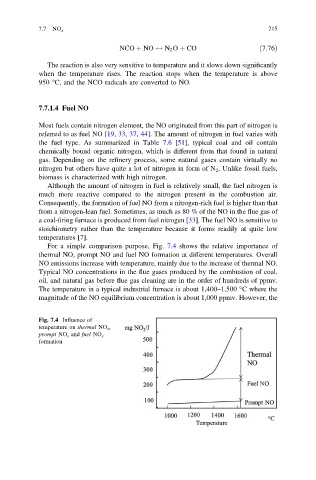
For a simple comparison purpose, Fig. 7.4 shows the relative importance of
thermal NO, prompt NO and fuel NO formation at different temperatures. Overall
NO emissions increase with temperature, mainly due to the increase of thermal NO.
Typical NO concentrations in the flue gases produced by the combustion of coal,
oil, and natural gas before flue gas cleaning are in the order of hundreds of ppmv.
The temperature in a typical industrial furnace is about 1,400–1,500 °C where the
magnitude of the NO equilibrium concentration is about 1,000 ppmv. However, the
Fig. 7.4 Influence of
temperature on thermal NO x ,
prompt NO x and fuel NO x
formation