Page 378 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 378
12.4 Carbon Capture Processes 357
C sðÞ þ CO 2 ! 2CO Boudouard reaction ð12:10Þ
Partial oxidation of methane can be described as
1
CH 4 þ O 2 ! CO + 2H 2 Methane partial oxidation ð12:11Þ
2
Steam methane reaction
CH 4 þ H 2 O ! CO + 3H 2 ð12:12Þ
The steam-methane reaction is effective in the temperature range of 700–950 °C
at 1.4–4 MPa. Under these conditions, 70–80 % of the methane can be converted
into hydrogen in one single reactor.
The syngas can be directly burned for energy production where CO is converted
into CO 2 by oxidation, but water–gas shift (WGS) is necessary to facilitate pre-
combustion carbon capture. The CO produced in partial oxidation process even-
tually is converted into CO 2 by the WGS reaction
CO + H 2 O ! H 2 þCO 2 ð12:13Þ
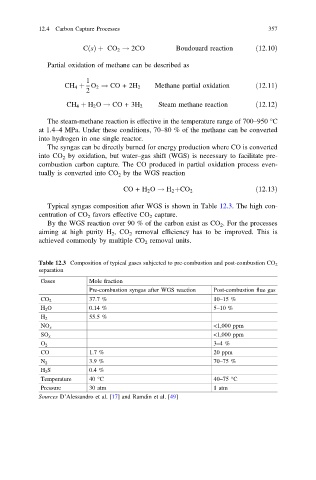
Typical syngas composition after WGS is shown in Table 12.3. The high con-
centration of CO 2 favors effective CO 2 capture.
By the WGS reaction over 90 % of the carbon exist as CO 2 . For the processes
aiming at high purity H 2 ,CO 2 removal efficiency has to be improved. This is
achieved commonly by multiple CO 2 removal units.
Table 12.3 Composition of typical gases subjected to pre-combustion and post-combustion CO 2
separation
Gases Mole fraction
Pre-combustion syngas after WGS reaction Post-combustion flue gas
CO 2 37.7 % 10–15 %
H 2 O 0.14 % 5–10 %
55.5 %
H 2
NO x <1,000 ppm
SO x <1,000 ppm
3–4%
O 2
CO 1.7 % 20 ppm
N 2 3.9 % 70–75 %
H 2 S 0.4 %
Temperature 40 °C 40–75 °C
Pressure 30 atm 1 atm
Sources D’Alessandro et al. [17] and Ramdin et al. [49]