Page 391 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 391
370 12 Carbon Capture and Storage
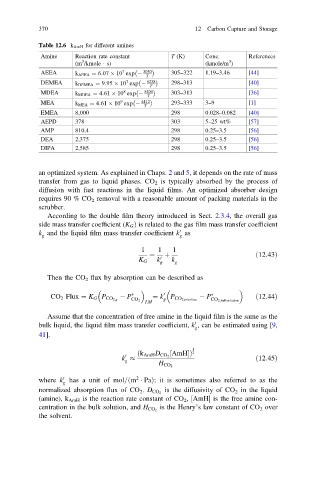
Table 12.6 k AmH for different amines
Amine Reaction rate constant T (K) Conc. References
3
3
(m /kmole · s) (kmole/m )
7
AEEA k AEEA ¼ 6:07 10 exp 3030 305–322 1.19–3.46 [44]
T
7
DEMEA k DEMEA ¼ 9:95 10 exp 6238 298–313 [40]
T
8
MDEA k MDEA ¼ 4:61 10 exp 5400 303–313 [36]
T
9
MEA k MEA ¼ 4:61 10 exp 4412 293–333 3–9 [1]
T
EMEA 8,000 298 0.028–0.082 [40]
AEPD 378 303 5–25 wt% [57]
AMP 810.4 298 0.25–3.5 [56]
DEA 2,375 298 0.25–3.5 [56]
DIPA 2,585 298 0.25–3.5 [56]
an optimized system. As explained in Chaps. 2 and 5, it depends on the rate of mass
transfer from gas to liquid phases. CO 2 is typically absorbed by the process of
diffusion with fast reactions in the liquid films. An optimized absorber design
requires 90 % CO 2 removal with a reasonable amount of packing materials in the
scrubber.
According to the double film theory introduced in Sect. 2.3.4, the overall gas
side mass transfer coefficient (K G Þ is related to the gas film mass transfer coefficient
0
k and the liquid film mass transfer coefficient k as
g g
1 1 1
¼ þ ð12:43Þ
K G k 0 k
g g
Then the CO 2 flux by absorption can be described as
P 0 P ð12:44Þ
g
CO 2 Flux ¼ K G P CO 2;g ¼ k P CO 2;interface
CO 2 CO 2;bulksolution
LM
Assume that the concentration of free amine in the liquid film is the same as the
0
bulk liquid, the liquid film mass transfer coefficient, k , can be estimated using [9,
g
41].
1
ð k AmH D CO 2 ½ AmHÞ 2
0
k ð12:45Þ
g
H CO 2
0 2
where k has a unit of mol= m Pað Þ; it is sometimes also referred to as the
g
is the diffusivity of CO 2 in the liquid
normalized absorption flux of CO 2 . D CO 2
(amine), k AmH is the reaction rate constant of CO 2 , AmH½ is the free amine con-
is the Henry’s law constant of CO 2 over
centration in the bulk solution, and H CO 2
the solvent.