Page 387 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 387
366 12 Carbon Capture and Storage
12.6 CO 2 Separation by Absorption
CO 2 absorption can also be classified into physical and chemical absorptions.
Physical absorption requires much lower energy to regenerate the solvent than
chemical absorption. A conceptual comparison between chemical and physical
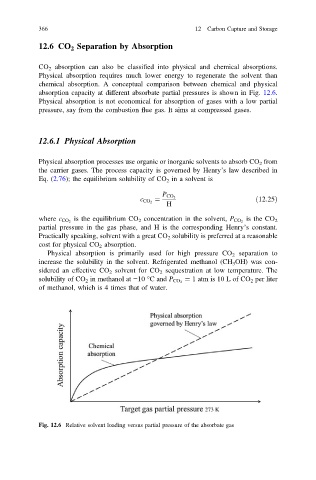
absorption capacity at different absorbate partial pressures is shown in Fig. 12.6.
Physical absorption is not economical for absorption of gases with a low partial
pressure, say from the combustion flue gas. It aims at compressed gases.
12.6.1 Physical Absorption
Physical absorption processes use organic or inorganic solvents to absorb CO 2 from
the carrier gases. The process capacity is governed by Henry’s law described in
Eq. (2.76); the equilibrium solubility of CO 2 in a solvent is
P CO 2
¼ ð12:25Þ
c CO 2
H
where c CO 2 is the equilibrium CO 2 concentration in the solvent, P CO 2 is the CO 2
partial pressure in the gas phase, and H is the corresponding Henry’s constant.
Practically speaking, solvent with a great CO 2 solubility is preferred at a reasonable
cost for physical CO 2 absorption.
Physical absorption is primarily used for high pressure CO 2 separation to
increase the solubility in the solvent. Refrigerated methanol (CH 3 OH) was con-
sidered an effective CO 2 solvent for CO 2 sequestration at low temperature. The
¼ 1 atm is 10 L of CO 2 per liter
solubility of CO 2 in methanol at −10 °C and P CO 2
of methanol, which is 4 times that of water.
Fig. 12.6 Relative solvent loading versus partial pressure of the absorbate gas