Page 470 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 470
15.2 Source Sampling 451
Aerosol Sampling
particles
U probe
0
Over
U >U isokinetic
s 0
sampling
Aerosol Sampling
particles
U probe
0
Under
U <U isokinetic
s 0
sampling
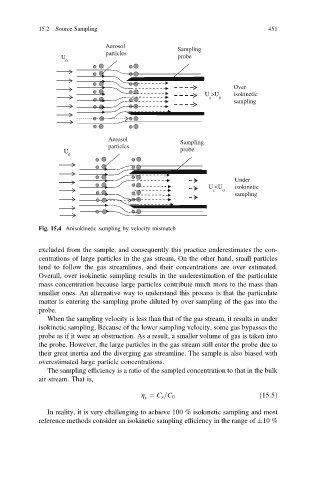
Fig. 15.4 Anisokinetic sampling by velocity mismatch
excluded from the sample, and consequently this practice underestimates the con-
centrations of large particles in the gas stream. On the other hand, small particles
tend to follow the gas streamlines, and their concentrations are over estimated.
Overall, over isokinetic sampling results in the underestimation of the particulate
mass concentration because large particles contribute much more to the mass than
smaller ones. An alternative way to understand this process is that the particulate
matter is entering the sampling probe diluted by over sampling of the gas into the
probe.
When the sampling velocity is less than that of the gas stream, it results in under
isokinetic sampling. Because of the lower sampling velocity, some gas bypasses the
probe as if it were an obstruction. As a result, a smaller volume of gas is taken into
the probe. However, the large particles in the gas stream still enter the probe due to
their great inertia and the diverging gas streamline. The sample is also biased with
overestimated large particle concentrations.
The sampling efficiency is a ratio of the sampled concentration to that in the bulk
air stream. That is,
g ¼ C s =C 0 ð15:5Þ
s
In reality, it is very challenging to achieve 100 % isokinetic sampling and most
reference methods consider an isokinetic sampling efficiency in the range of 10 %