Page 76 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 76
50 2 Basic Properties of Gases
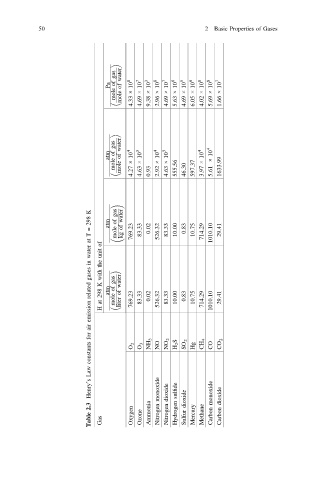
gas water
Pa of of 10 8 10 7 10 3 10 8 10 7 10 6 10 5 10 6 10 8 10 8 10 7
mole mole × × × × × × × × × × ×
4.33 4.69 9.38 2.96 4.69 5.63 4.69 6.05 4.02 5.69 1.66
gas water
atm of of 10 4 10 3 10 4 10 3 10 4 10 4
mole mole × × × × 555.56 597.37 × × 1633.99
4.27 4.63 0.93 2.92 4.63 46.30 3.97 5.61
gas
K water
298 atm of
= of 769.23 83.33 0.02 526.32 83.33 10.00 0.83 10.75 714.29 29.41
T mole kg 1010.10
at of
water unit
in the
gases with gas water
related K 298 atm of mole of 769.23 83.33 0.02 526.32 83.33 10.00 0.83 10.75 714.29 1010.10 29.41
emission at H liter
air
for NH 3 NO 2 H 2 S SO 2 CH 4 CO 2
constants O 2 O 3 NO Hg CO
Law
Henry’s monoxide dioxide sulfide monoxide dioxide
2.3 Ammonia Hydrogen dioxide
Table Gas Oxygen Ozone Nitrogen Nitrogen Sulfur Mercury Methane Carbon Carbon